| Pages:
1
2 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Air rifle pump for pressurizing catalytic hydrogenation vessel?
This pump seems to be a fairly inexpensive solution for pressurizing a small-to-medium sized pressure vessel:
http://www.ebay.com/itm/252638562648
It seems to be able to attach a gas cylinder to its inlet too. It seems like it could be used to transfer hydrogen from a lower-pressure generation
vessel to a higher-pressure reaction vessel. I've wanted to try using various pressurized reactions up to 10 atmospheres or so, and this seems like
it could go well beyond that. On the other hand, pressurized reactions seem to be either of the "several atmospheres" variety or the "core of
Jupiter" variety, without that much in between.
Is there anything significant that I'd be able to do at 1000 psi that wouldn't be possible at 100? Specifically, small-scale catalyzed reactions?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I think this a good find! I'm going to do some hydogenation, starting at atmospheric pressure. But I may want to increase the pressure at some
point.
A bladder will be needed to accumulate the H2 before it is pressurized. Any ideas what could be used other than a plastic bag?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Am I thinking correctly? 4500 psi is about 300 atmospheres...what will hold this? And a good bicycle pump, considerably less expensive will fill
something to ~8 atmospheres? Would an amateur really want any more pressure than this? Here in my town, yesterday a 1 ton boiler blew up and was
hurled 500 feet through the roofs of two buildings, killing 4 and injuring a couple of other people. (I don't know what at pressure the boiler was
operating.)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Magpie  |
A bladder will be needed to accumulate the H2 before it is pressurized. Any ideas what could be used other than a plastic bag?
|
In my previous research, which I had forgotten, I felt that the Mylar balloons used for He should be good for low pressure accumulation of H2. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I'd probably generate hydrogen via Al/Ga/In/St alloy and water, then store it in a thick-walled garbage bag duct taped over my container opening.
Since the pressure in the bag would barely be above atmospheric, not much hydrogen should escape, as long as you maintained that low pressure in the
bag. But theoretically, you could create a hatch on one of those portable air tanks, and then put your hydrogen generation stuff inside the tank.
You could tell if it had pressure just by the pressure gauge that they always come with.
Quote: Originally posted by CharlieA  | | Am I thinking correctly? 4500 psi is about 300 atmospheres...what will hold this? And a good bicycle pump, considerably less expensive will fill
something to ~8 atmospheres? Would an amateur really want any more pressure than this? Here in my town, yesterday a 1 ton boiler blew up and was
hurled 500 feet through the roofs of two buildings, killing 4 and injuring a couple of other people. (I don't know what at pressure the boiler was
operating.) |
First off, the 4500 psi is probably WAY too optimistic. Keep in mind this is a Chinese knockoff of a US-made item. However, I'd expect it to be able
to do 3000-3500, even so. There's a gauge on it, specifically so you know where to stop. Secondly, most hydrogenation catalysts
aren't active until at least the tens of atmospheres pressures range. IIRC, only palladium, and to a lesser extent platinum, can do a significant
amount of hydrogenation at pressures attainable with a bicycle pump. Third, comparing a 1-liter vessel exploding to a one-ton boiler... that's
probably the silliest non-sequitur I've ever responded to. Not to mention, I doubt anyone here could reach the 4500 psi mark anyhow, because hydrogen
is so fantastic at escaping from any sort of apparatus designed to contain it under pressure.
Oh yeah, you wouldn't expect this to be the case, but Soda Stream plastic bottles are actually rated to 1000 psi!. Reason being, carbonation needs
those pressures to force the CO2 into solution quickly. When I realized how pressurized those things got, I started occasionally reading articles
that mentioned 500+ psi, whereas I never bothered to as soon as I'd see that it was done under that high of a pressure. But now, those reactions are
within the realm of possibility, using homemade apparatus.
[Edited on 4/5/17 by Melgar]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Ordinary cylinders of hydrogen are normally sold in pressures around 140-180 bar, and I carried out some hydrogenations at these pressures back in
grad school. I used a special cell to contain the reagents, and the department had a special armored room, in case hydrogen detonation occurred. (And
one actually did, driving a small bit of metal into my finger.)
But high pressure hydrogenation is not always needed, if you use the correct catalyst. Discoveries by Wilkinson and others starting back in the 60's
found organorhodium catalysts that could hydrogenate alkenes and alkynes at ambient pressure, with considerable regio- and stereospecificity.
[Edited on 4/5/17 by PirateDocBrown]
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Also, take into account two other things-hydrogen embrittlement of metals.
And Al/Ga/In/Sn would be a waste of a useful reducing agent, when a block of aluminium and some caustic soda solution can be used to generate H2 at a
steady rate. If a solid block of Al is used, one could even rig up something to raise and lower the block into the solution.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  |
Third, comparing a 1-liter vessel exploding to a one-ton boiler... that's probably the silliest non-sequitur I've ever responded to.
[Edited on 4/5/17 by Melgar] |
You may still end up dead if you are next to it when it goes.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by PirateDocBrown  | | Ordinary cylinders of hydrogen are normally sold in pressures around 140-180 bar, and I carried out some hydrogenations at these pressures back in
grad school. I used a special cell to contain the reagents, and the department had a special armored room, in case hydrogen detonation occurred. (And
one actually did, driving a small bit of metal into my finger.) |
That would be from hydrogen being compressed alongside oxygen or a strong oxidizer, no? In any case, the pressure vessel could be surrounded by a
pot, or a pail, or a jug, just to be safe. I'd want to use a plastic container for high pressure anyway, to minimize the danger of an explosion. And
for the plastic Soda Stream bottles I'd want to use, I'd probably only go up to about 50 bar anyway.
Quote: Originally posted by PirateDocBrown  | | But high pressure hydrogenation is not always needed, if you use the correct catalyst. Discoveries by Wilkinson and others starting back in the 60's
found organorhodium catalysts that could hydrogenate alkenes and alkynes at ambient pressure, with considerable regio- and stereospecificity.
|
I'm sure if I had a Sigma Aldrich account and infinite amounts of money, it'd be something worth considering. Alas, I have neither.
This pump would be operating at a fraction of its capacity, and the only metal parts in contact with the hydrogen would be the metal of the pump.
Additionally, hydrogen embrittlement is virtually unheard of at room temperature, and typically is a problem that arises during fabrication. I
actually did think of that already, but either temperatures or pressures would need to be orders of magnitude higher in order for that to come into
play.
Quote: Originally posted by tsathoggua1  | | And Al/Ga/In/Sn would be a waste of a useful reducing agent, when a block of aluminium and some caustic soda solution can be used to generate H2 at a
steady rate. If a solid block of Al is used, one could even rig up something to raise and lower the block into the solution. |
The prices aren't as different as you probably think they are. Gallium and indium can be purchased for about $0.50 / gram, and alloyed with aluminum
at 5% by weight. The alloy is also incredibly brittle, and can be broken by hand with the aid of a pair of pliers, making it very easy to use only as
much as you need. Also, it's easy to recycle the gallium and indium, if you want to reclaim it. Granted, the main reason I'd use it is because I
have a lot of it already, but there's no reason NaOH and aluminum wouldn't work. I've always used that and aluminum electric fence wire, cut into
pieces about 10 cm long, put in a bottle with maybe 4 cm of NaOH solution at the bottom.
I don't think there are many experiments that have been posted on these boards that couldn't kill a complete idiot with no regard for safety.
High pressures aren't inherently unsafe though, and I have a lot more experience using high-pressure equipment than I do with hydrogenation, so that
part doesn't really worry me.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | Quote: Originally posted by PirateDocBrown  | | Ordinary cylinders of hydrogen are normally sold in pressures around 140-180 bar, and I carried out some hydrogenations at these pressures back in
grad school. I used a special cell to contain the reagents, and the department had a special armored room, in case hydrogen detonation occurred. (And
one actually did, driving a small bit of metal into my finger.) |
That would be from hydrogen being compressed alongside oxygen or a strong oxidizer, no? In any case, the pressure vessel could be surrounded by a
pot, or a pail, or a jug, just to be safe. I'd want to use a plastic container for high pressure anyway, to minimize the danger of an explosion. And
for the plastic Soda Stream bottles I'd want to use, I'd probably only go up to about 50 bar anyway.
|
No, no oxidizers mixed in, just hydrogen. But hydrogen can self-ignite in air during explosive decompression. (And burns with colorless flame!)
In my case, the quantity of hydrogen was small, the effect was not unlike holding a small firecracker. The pressure cell had a metal release vent,
which acted as designed, but my hand just happened to be in the wrong place at the wrong time. The sliver was maybe 3 mm long, and zapped me in the
knuckle of my ring finger. It was pretty deep, but stopped by bone. I plucked it out with tweezers and applied Neosporin and a bandaid. Less than a
half hour of work time lost, I didn't even bother to fill out an accident report. Still have a cool scar though.
|
|
|
cabal
Harmless

Posts: 18
Registered: 10-4-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  | No, no oxidizers mixed in, just hydrogen. But hydrogen can self-ignite in air during explosive decompression. (And burns with colorless flame!)
In my case, the quantity of hydrogen was small, the effect was not unlike holding a small firecracker. The pressure cell had a metal release vent,
which acted as designed, but my hand just happened to be in the wrong place at the wrong time. The sliver was maybe 3 mm long, and zapped me in the
knuckle of my ring finger. It was pretty deep, but stopped by bone. I plucked it out with tweezers and applied Neosporin and a bandaid. Less than a
half hour of work time lost, I didn't even bother to fill out an accident report. Still have a cool scar though. |
Hey, I'd just like to correct you on one minor detail: a mixture of hydrogen and oxygen doesn't self-ignite. It's just that the energy needed to
ignite it is so minor (don't quote me on this, it's something like 0,01-0,02 mJ) that even the electrostatic charge developed by a particle of rust
falling through the mixture can be enough to ignite it.
Anyway, Melgar, I like the idea of the pump. Did you go through with it?
How did it turn out?
Just a couple thoughts: You should try to absolutely minimize the amount of air that gets pumped into your hydrogenator. Not just because of the
safety concerns that PirateDocBrown mentioned, but also to make sure that your hydrogenation isn't poisoned by any particles in the air.
What does your hydrogenator look like?
I'm assuming that you have two valves on your vessel, one leading to your vacuum pump, the other to your hydrogen source (i.e. your air pump). So the
best course of action would be to completely evacuate the whole vessel while making sure that the valve that connects to the tubing of the air pump is
open. This way, you also evacuate the air that is inside the tubing. Then close the vacuum valve and start pumping the hydrogen in there until the
manometer shows the desired pressure. Then close the valve to the pump. I'm not sure how the air pump is constructed and whether it construct to
withstand the vacuum, but that's how I'd try it. For optimal safety, do a trial run just with compressed air inb4 so you can check for leaks (put
soapy water on all joints or any possible leak and then pump it up - if there's bubbling, you got a leak).
This sounds like a cool way to do some DIY hydrogenations without the need to buy a whole cylinder of the stuff. I just got one, and while they're
cheap and readily available, I now have more than a thousand liters of H2, the majority of which I probably won't even use. Plus I need to
get it checked regularly and have outside storage to prevent gas buildup etc etc.
P.S.: Remember to also evacuate the container where you're producing your hydrogen when you're filling up the balloon.
[Edited on 10-5-2017 by cabal]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by cabal  |
This sounds like a cool way to do some DIY hydrogenations without the need to buy a whole cylinder of the stuff. I just got one, and while they're
cheap and readily available...
|
Where did you get this cylinder? What size is it? How much did it cost? I assume you have to have a pressure regulator. Was there any hassle when
buying it - like paperwork for intended use, etc?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by cabal  | Anyway, Melgar, I like the idea of the pump. Did you go through with it?
How did it turn out?
Just a couple thoughts: You should try to absolutely minimize the amount of air that gets pumped into your hydrogenator. Not just because of the
safety concerns that PirateDocBrown mentioned, but also to make sure that your hydrogenation isn't poisoned by any particles in the air.
What does your hydrogenator look like?
I'm assuming that you have two valves on your vessel, one leading to your vacuum pump, the other to your hydrogen source (i.e. your air pump). So the
best course of action would be to completely evacuate the whole vessel while making sure that the valve that connects to the tubing of the air pump is
open. This way, you also evacuate the air that is inside the tubing. Then close the vacuum valve and start pumping the hydrogen in there until the
manometer shows the desired pressure. Then close the valve to the pump. I'm not sure how the air pump is constructed and whether it construct to
withstand the vacuum, but that's how I'd try it. For optimal safety, do a trial run just with compressed air inb4 so you can check for leaks (put
soapy water on all joints or any possible leak and then pump it up - if there's bubbling, you got a leak).
This sounds like a cool way to do some DIY hydrogenations without the need to buy a whole cylinder of the stuff. I just got one, and while they're
cheap and readily available, I now have more than a thousand liters of H2, the majority of which I probably won't even use. Plus I need to
get it checked regularly and have outside storage to prevent gas buildup etc etc.
P.S.: Remember to also evacuate the container where you're producing your hydrogen when you're filling up the balloon. |
I got the pump, but haven't built the hydrogenation vessel yet. The quick-disconnect adapter seems to be non-standard, and I need to figure out where
to get connectors that will fit with it. That shouldn't be too hard, it's just a matter of having the time to do it.
I have drawn up plans for it though. The bottle only has one inlet, which is on the cap, and has a tube leading down to the bottom of the vessel.
When I want to purge it, I'd just screw on the cap loosely and pump a few liters of hydrogen into it. Then screw on the cap tightly and start
pressurizing it.
As far as removing oxygen, I've been using antioxidants a lot lately, especially ascorbic acid, and I plan to start using BHT as well, for suppressing
peroxide formation in ethers among other things. After doing as much as possible to remove oxygen from my gas stream, the plan was to use small
amounts of one of these antioxidants just to be safe.
The pump can only work over a gradient of about 50 bar though, and can only reach its maximum rated pressure when pumping from a pressurized tank.
That's fine, I suppose, since that's about 750 psi, and my bottle is only rated for 1000. I just need to find reactions and catalysts that will work
at that pressure range.
|
|
|
cabal
Harmless

Posts: 18
Registered: 10-4-2017
Member Is Offline
Mood: No Mood
|
|
When you're talking about the adapter of the air pump, do you mean the one at the end of the tubing or the one where you'll attach your H2
bag? Because if it's the former, you could just cut it off, put a stainless steel fitting with a nozzle on top of your hydrogenator and then clamp the
tubing on tight. That'll probably work fine for any low-pressure procedure.
By the way: how large is your hydrogenator? You were talking about 50 bar before - even if your vessel is only a couple hundred ml, that'd be one big
bag of hydrogen.
Concerning the safety: While the antioxidants certainly won't hurt, they'll not take care of the highly combustible hydrogen/air mixture. Keep in mind
that if it contains less than 24% air, the gas mixture will not be combustible. Try to calculate the respective amounts. However, if you start with a
non-evacuated vessel and you push the gas out, you'll have some combustible gas coming out either way. Keep in mind that hydrogen is a lot lighter
than air, so it might have a hard time pushing the air out.
Concerning the reactions: Finding interesting reactions and catalysts that will work at low pressure won't be much of a problem, I think. I've just
read Morris Freifelder's Catalytic Hydrogenation in Organic Synthesis Procedures and Commentary, and it's chock full of reactions that work
within a 1-5 atm pressure range. Let me know if you'd like to have it, I photocopied the chapters that were of interest to me. I also got a couple
more recent books if you're interested.
In one of them, I found a seemingly easy way to produce a platinum catalyst that is a good choice for many of the reactions that Freifelder describes.
I'll make a thread about it soon.
Hopefully you'll get around to constructing the apparatus. I'd like to see how it looks and whether it works.
Quote: Originally posted by Magpie  | | Where did you get this cylinder? What size is it? How much did it cost? I assume you have to have a pressure regulator. Was there any hassle when
buying it - like paperwork for intended use, etc? |
I called the local gas supplier and made an order, bought
the regulator, tubing, safety gear right with the cylinder. Cost me a little over 200 bucks, and I can probably get a hundred of that back when I sell
the cylinder. No hassle or paperwork at all. But I'm European, so YMMV.
[Edited on 10-5-2017 by cabal]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
| Quote: | | When you're talking about the adapter of the air pump, do you mean the one at the end of the tubing or the one where you'll attach your H2 bag?
Because if it's the former, you could just cut it off, put a stainless steel fitting with a nozzle on top of your hydrogenator and then clamp the
tubing on tight. That'll probably work fine for any low-pressure procedure. |
I designed the thing to go up to at least 50 atmospheres. Is that considered low? In any case, I really WOULD like to use the quick-disconnect
fittings, since that way I could easily attach the vessel to the pump and H2 source, then disconnect it to stir it or otherwise agitate it. Not to
mention, I could theoretically compress hydrogen in a tank, then pressurize my vessel from that tank after it hit its limit for pumping from
atmospheric pressure. If I needed exercise, I could even do it in several stages, assuming I had tanks that were rated for it. So I'd rather not
make any modifications that would limit its future capacity too much.
| Quote: | | By the way: how large is your hydrogenator? You were talking about 50 bar before - even if your vessel is only a couple hundred ml, that'd be one big
bag of hydrogen. |
It's 1000 mL. The idea is that I'd start generating hydrogen and pumping at about the same time. So as its filling, I'm constantly removing it. I'd
estimate volume by counting strokes, I suppose. My friend has a hydrogen generator for an oxyhydrogen torch as well, if generating hydrogen
chemically proves to be cumbersome.
| Quote: | | Concerning the safety: While the antioxidants certainly won't hurt, they'll not take care of the highly combustible hydrogen/air mixture. Keep in mind
that if it contains less than 24% air, the gas mixture will not be combustible. Try to calculate the respective amounts. However, if you start with a
non-evacuated vessel and you push the gas out, you'll have some combustible gas coming out either way. Keep in mind that hydrogen is a lot lighter
than air, so it might have a hard time pushing the air out. |
If I pump fast enough that the flow is turbulent, then it'll mix thoroughly enough that the gas coming out will be representative of its
concentrations inside the vessel. That is, the different gases won't have time to stratify according to density, since there would be too much
turbulence. Woohoo, fluid mechanics was finally useful for something!
| Quote: | | Concerning the reactions: Finding interesting reactions and catalysts that will work at low pressure won't be much of a problem, I think. I've just
read Morris Freifelder's Catalytic Hydrogenation in Organic Synthesis Procedures and Commentary, and it's chock full of reactions that work within a
1-5 atm pressure range. Let me know if you'd like to have it, I photocopied the chapters that were of interest to me. I also got a couple more recent
books if you're interested. |
I'm interested in reducing nitriles (and various aliphatic nitro groups) to amines, since nitriles are easy to come by for testing, and I'm pretty
sure catalytic hydrogenation is the most commonly-cited way to do that particular reduction. I guess I'm also looking for reactions that can be done
catalytically under pressure, that can't be done more easily some other way.
Also, reactions that use nickel or copper (which I have a lot of), ruthenium (which I have a decent amount of), palladium (which I have a few grams
of), and possibly rhodium (which I have half a gram of, as its sulfate salt. Obviously, I'd want to start with stuff that I didn't care as much if it
was wasted, so I guess base metals and ruthenium?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Seems to me, the reduction of Nitriles to Amines isn't especially difficult. Gotta use the right catalyst, and be aware of the fact that alternate
products may be possible, depending on reaction conditions. Fer instance, when reducing Acetonitrile, the principle product may prove to be
Di-ethylamine, rather than Ethylamine. Your Ethylamine can react, once formed, with an imine(aldehyde) intermediate, and be reduced to mostly
Diethylamine.
To be considered, would be hydrogenations utilizing WC as a catalyst. Under acetic condition, utilizing moderate heat and pressures, Tungsten Carbide
wakes up and becomes a nifty catalyst for reducing Nitro groups.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, I wanted to start with something easy for that reason. To make sure it worked as intended and get a better sense of the reaction's
requirements.
| Quote: | | Gotta use the right catalyst, and be aware of the fact that alternate products may be possible, depending on reaction conditions. Fer instance, when
reducing Acetonitrile, the principle product may prove to be Di-ethylamine, rather than Ethylamine. Your Ethylamine can react, once formed, with an
imine(aldehyde) intermediate, and be reduced to mostly Diethylamine. |
I remember reading that when reducing nitro or nitrile groups to primary amines, it's good for there to be some ammonia present, because then ammonia
can get alkylated instead of an amine, which would give the same product. Also, I remember reading to use acidic conditions if possible so that
amines form salts, which are less susceptible to alkylation. Is this more or less accurate, assuming compatibility with other functional groups and
the catalyst?
| Quote: | | To be considered, would be hydrogenations utilizing WC as a catalyst. Under acetic condition, utilizing moderate heat and pressures, Tungsten Carbide
wakes up and becomes a nifty catalyst for reducing Nitro groups. |
Well, now that I've found a source for it, I can see it's not cheap, but still way cheaper than PGMs. The articles I've found on it seem to use it as
a support for palladium though, and so far I haven't found much on what temperatures it takes to activate it. I'm using a PET bottle to start out, so
I can see what's going on inside, and also because of this material's remarkably high strength at temperatures below 100C or so. I'll keep reading up
on tungsten carbide though, it sounds interesting.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
What? You haven't read this?
I'd ditch the PET, but other than that.....Most guys can manage to generate a few atmospheres, at under 150c, under acetic conditions.
http://www.google.ch/patents/US5646085
Considered this approach for hydrogenating Nitro-olefins. Though I suspect, temperatures are a little high and might encourage polymerization.
Apparently, these catalysts resist poisoning......something you will grow to appreciate, as you go along.
Was hoping even more active catalyst-systems might be reported, as the extreme hydrogen adsorbing qualities of Nanotubes and carbonized feathers were
discovered. Haven't read about such experiments. But then, if you were a large corporation, and you had developed a revolutionary proprietary
process.......Would you be hot to let the cat out of the bag? Or, would you prefer to quietly cash in on your competitive advantage?
So.....we don't really know, what's actually out there.
Got some examples in the actual patent pages. Pretty good procedure for producing aniline.
The micro-structure of some WC catalysts and Pt catalysts, are quite similar. Excepting WC is dirt cheap, somewhat less active, and it resists
poisoning.
[Edited on 21-5-2017 by zed]
[Edited on 21-5-2017 by zed]
[Edited on 21-5-2017 by zed]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Oh, I see. AROMATIC nitro groups. Like there aren't already a million patents out there on reducing nitroarenes. I mean, you can throw just about
any metal powder into an aqueous or water/alcohol solution containing acid and your nitroarene, and it'll get reduced to the corresponding aniline.
From how many PhD dissertations have been written on new ways to reduce a nitroarene, I'm starting to suspect that it's the simplest reduction that
it's possible to do in organic chemistry. Of course, that's a long ways from reducing nitroalkanes or nitroalkenes.
| Quote: | | Considered this approach for hydrogenating Nitro-olefins. Though I suspect, temperatures are a little high and might encourage polymerization.
|
Well, I'll save you some trouble: it won't work. Nitroarenes practically reduce themselves; nitroolefins are a bit trickier.
| Quote: | | The micro-structure of some WC catalysts and Pt catalysts, are quite similar. Excepting WC is dirt cheap, somewhat less active, and it resists
poisoning. |
I didn't quote the rest of what you said, just because I'd have responded "sounds great, except that it won't work for anything but nitroarenes" to
everything you said. Believe me, there is NO shortage of ways to reduce nitroarenes out there.
Bummer, well, I'm glad I didn't buy any tungsten carbide anyway. It sounded a bit too good to be true.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Buy a pre-prepared WC catalyst? Don't know about that. I don't think I'd do it. Catalysts often have a poor shelf-life.
The authors of the above patent, instruct us on how to make an active WC catalyst.
This catalyst exhibits qualities very similar to Pt/Pd, and it is similarly inert to chemical attack. Could be really useful in some applications.
Whether or not, this catalyst will perform your particular reduction remains to be seen.
[Edited on 23-5-2017 by zed]
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
There's something you should keep in mind when trying to handle hydrogen with equipment designed for other gases. Hydrogen will leak through
connections that are gas tight for larger molecules. I have some question about how well that air rifle pump could possibly work for hydrogen.
Although I suppose if leakage isn't a huge issue, you could probably achieve some pressurization.
[Edited on 5/23/2017 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Doc B
Hazard to Others
  
Posts: 107
Registered: 5-4-2012
Member Is Offline
Mood: No Mood
|
|
Did anyone get this pump and try to compress hydrogen or other gasses except air?
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I do have the pump, and it seems to be pretty solid. Comes in a nice carrying case, with several connectors that could be used in different
configurations.
I actually just purchased some smaller 500-mL PETE bottles that are rated to 1000 psi at least. I tried to find the right fitting for the
quick-disconnect joint, but they only had 1/4 inch pneumatic joints at the hardware store. This one is 8mm, and probably only realistically available
via a foreign supplier. The caps of these bottles are all plastic, and I'm doubtful I could drill a hole in one while preserving its structural
integrity.
I'm considering having a metal cap machined, but the friend of mine that I'd get it through has been really unreliable lately, and hasn't followed
through on anything he said he'd do. He just keeps asking me to mail him some stuff he needs first. Somehow in two weeks he hasn't been able to come
up with $20 to ship the package, and no way I'm ever going to send him cash, so I had to jump through a few hoops to generate a shipping label that he
can print and attach to the package. Sorry for the aside. Not having much money, and having to rely on the least reliable person I know, kind of
sucks.
I'm not really worried about the pump, since its internals are rated to 30 MPa for air. Probably less for hydrogen, but I have to assume that there's
a linear correlation between resistance to air loss and resistance to hydrogen loss, even if they're different numbers. My main concern right now is
getting a stronger cap that can be fitted with a pressure gauge and a quick-disconnect inlet.
I did make some Pd/C catalyst, although I used activated charcoal that was meant for a fishtank filter. I did treat it with nitric acid first though.
I'd also like a simple reaction that's easy to test for the desired product. I was thinking acetonitrile to ethylamine might be a good choice,
because nitrile reduction to amines seem to be easy to do with Pd/C, but significantly more difficult with other reducing agents that I might have
lying around. If anyone has any other suggestions, I'd be happy to hear them, assuming that the reaction is well-documented, (ie, not just one
patent) and the catalyst isn't something that looks like a mutated version of this:
[Edited on 6/12/17 by Melgar]
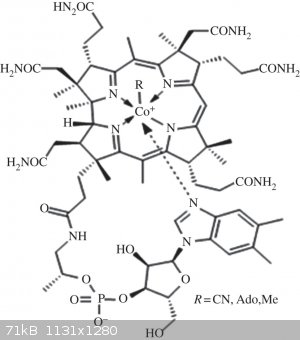
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
How does this cap attach? Screwed on, I presume.
Could you show a picture of the cap. Maybe I can help here.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by Magpie  | How does this cap attach? Screwed on, I presume.
Could you show a picture of the cap. Maybe I can help here. |
OD of bottle opening is 35 mm. ID is 30 mm. Pitch is 4 mm. Lead is 8 mm (double start). Threads are flat on the side that the force would be pushing
against, sloped on the other side. Thread cut (height) is about 1.5 mm, but hard to measure accurately. Cap is 20 mm deep, plus the gasket thickness.
Lower (flat) side of the thread begins 9 mm past opening, and each of the threads makes one full rotation. (Ends 17 mm past opening.) There is a
removeable gasket that seems to be silicone, but I haven't measured it yet.
Also including a picture of the pneumatic connectors that came with it. I need a quick-couple female connector that can attach to the second one,
which is an adapter for the hose that came with it.

[Edited on 6/12/17 by Melgar]
[Edited on 6/12/17 by Melgar]

The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
| Pages:
1
2 |
|