blablabla1
Harmless

Posts: 5
Registered: 5-5-2017
Member Is Offline
Mood: No Mood
|
|
Why is glacial acetic acid needed in HOCl-oxidation of sec.OH?
Hi, i'm looking at the mechanism of the oxidation of secondary alcohols and i'm wondering, why glacial acetic acid is needed?
the HOCl and the AcOH are forming the ClOH+, which is attacking the sec. acohol.
so far so good, bis isn't the HOCl mostly used in 5% water-solutions?
Does it just take langer if you use loweder AcOH, or does it interfere somewhere?
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
This is a "Beginnings" question.
Have a look at this video:
https://www.youtube.com/watch?v=CyRlSBrD4wI
Takes five seconds to find.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I think you're getting confused here. Stevens oxidation is the use of NaOCl and AcOH to selectively oxidise 2* alcohols to the corresponding ketones.
HOCl is formed in situ as the active oxidant
|
|
|
blablabla1
Harmless

Posts: 5
Registered: 5-5-2017
Member Is Offline
Mood: No Mood
|
|
thanks for the answers, the mechanism in the video is clear to me. i didtn think this is a beginners questions;
but still i found lots of procedures, where "5% NaOCl" solution should be used.
(maybe i should know this, i'm a chemistrys masters' for four yours know, but mainly inorganic...)
also, the video just expained the mechanism again :X )
[Edited on 5-5-2017 by blablabla1]
soooo well, the water would destroy the ClO- formed in situ, but why are there still so many (from university handbooks) instructions to use "5% HOCl bleach"?
does is in some cases just work?
[Edited on 5-5-2017 by blablabla1]
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Sorry. 
Having a closer look at the mechanism, it seems unlikely the sec. alcohol is protonated by HOCl as acetic acid is ~1000 times stronger acid. OCl⁻ is
a better nucleophile than expected as there is an alpha effect. Occasionally mechanisms are just proposed, suggestions looking good in the first place.
EDIT:
I'm not sure what your problem is. In the procedure linked, NaOCl is used. About 2 eq. AcOH are added:
NaOCl + AcOH ---> HOCl + NaOAc
The resulting mixture contains: HOCl, AcOH and NaOAc.
[Edited on 5-5-2017 by Alice]
|
|
|
blablabla1
Harmless

Posts: 5
Registered: 5-5-2017
Member Is Offline
Mood: No Mood
|
|
i have found another mechanism, but since i've been playing with metalcomplexes for 3 years now, i don't know shit about these stuff (allwhtough i
should :/ )
I found a mechanism, where the HOCl attacks the alcohol, forming Cl-O+H2 and AcO-.
The chlorine would then attack a lone pair of the target sec. alcohol, giving free the attached H2O and adding a positive charge to the
alcoholic-O.
The Hydrogen at the alcohol is then taken by the AcO- from former and we have got a "RR-O-Cl".
This should then be attacked by water, taking away the acidid hydrogen at the oxygen, forming a keton an throwing out the chlorine.
so in the end, it sums up, but this mechanism is a bit fishy to me...
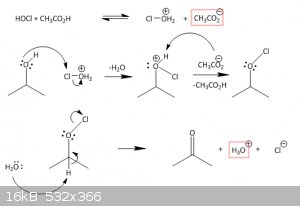
i think i'm gonna get my org chem books out again *dust off*
so just that i get it right:
1. the sec alc is protonated by HOCl due to the alpha effect.
2. the addition of OCl- is clear
3. catching of the Hydrogen, leaving of Cl- and forming a ketone is also clear.
so stillt, why can't there be any water, especially if i find a lot of (university) instructions, to use "5% NaOCl" Solution.
[Edited on 5-5-2017 by blablabla1]
[Edited on 5-5-2017 by blablabla1]
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
I'd say the second mechanism is plausible as well. 
At least this is what happens when hypochlorite reacts with amines. In that case there is no chance for the other mechanism.
|
|
|
blablabla1
Harmless

Posts: 5
Registered: 5-5-2017
Member Is Offline
Mood: No Mood
|
|
Hm, my amines are monstly protected, also i dont treat them with HOCl (also no air or water  )... i think it would wreck up the amine, forming NH3 or something you dont want to have in yout glovebox? )... i think it would wreck up the amine, forming NH3 or something you dont want to have in yout glovebox?
I'll just have to explain a class this oxidation soon, and the questions why there can't be water bothers me. (What they hear... well, IF they listen,
they dont care...)
I'm already afraid of questions about other functional groups around the alcohol 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm not sure about the mechanism of the formation of the alkyl hypochlorite, but I'd say there's no chance of the acetic or hypochlorous acid
protonating the hydroxyl of the alcohol as one of the steps. I favour the first one.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I think I see your difficulty. This reaction does not require anhydrous conditions - "glacial acetic acid" (acetic acid to us organic chemists) is
used because thats how it comes - a neat liquid rather than an aqueous solution.
The reaction would proceed equally well with aqeuous acetic acid; it is added to buffer the (basic) hypochlorite solution to a slightly acidic pH
where HOCl can exist in equilibrium.
[Edited on 5-5-2017 by DJF90]
|
|
|
blablabla1
Harmless

Posts: 5
Registered: 5-5-2017
Member Is Offline
Mood: No Mood
|
|
Thanks for your answer DFJ90, i already found some site that showed the HOCl <-> H+ + -OCL equilibrium and therefore
needing some acid to have more OCl- reagent.
Well i have to wait for a precursor to finish, so why lets try to make some acetone this way with 25% acetic acid... 
another quick question: the reaction of HOCl with amines is one of the "cleaning" effects i guess, since it chlorinates mono-amines and therefore
makes them "harmless"? arent secondary amines (chlorinated also) not dangerous,too (are are they chlorinated to R-NCl2?)
I know some, i wouldn't want to have in my bathroom...
[Edited on 5-5-2017 by blablabla1]
[Edited on 5-5-2017 by blablabla1]
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Rethinking the mechanism, it is indeed unlikely the alcohol is protonated in one way or another. It will be protonated to a minor degree, but then
OCl⁻ as another minor species (pKa HOAc vs. pka HOCl ) would have to attack. This would lead to an incredibly slow reaction.
Much more likely that HOCl delivers Cl⁺ as a strong electophile being attacked by the alcohol. Something known for many other reactions involving
HOCl. In HOCl the Cl is even more electrophilic compared to OCl⁻ and again more for H2OCl⁺.
@blablabla1. I think the hypochlorite in cleaning agents is used as a disinfectant in the first place, it chlorinates amines, not to make them
harmless but to make bacteria harmless. 
EDIT: And sorry again, that really wasn't my brightest moment....
[Edited on 5-5-2017 by Alice]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I believe the disinfecting effect that bleach has is primarily a result of the strong oxidizing properties of -OCl, as opposed to any of the effects
of HOCl. Sure, you'll get a bit of chloramine from that, but not very much, and not enough to do any real damage. Assuming pH is basic while it's
doing its work, the organic groups on any nitrogen molecules would probably all be oxidized away to CO2 and water. I know that if you leave anything
glass with crusty residue all over it in bleach, it will oxidize it away unless it consisted mostly of amines, in which case it will oxidize most of
it away and leave a bunch of yellow-green chloramine residue. Although chloramine is bad stuff when concentrated, it's the main thing you smell at
swimming pools, and they actually use the stuff in small quantities to chlorinate tap water, so it can't be that bad.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I actually question whether chloramine is the main thing you smell at swimming pools. I've smelled a pretty wide variety of swimming pools, halogens,
and halogenated substances, and I think the smell at club swimming pools is chlorate. Pools that get used by a lot of children like high school pools
have a different smell, which I assume is probably chloramines.
Halogens - have a sharp smell that is not altogether unpleasant until it is burning your nostrils
Halogenates - have a swimming pool smell that is not really unpleasant either. I kind of like it, actually.
Hypochlorite - has an unpleasant bleach smell
|
|
|
Nicodem
|
Thread Moved
6-5-2017 at 12:34 |