| Pages:
1
2
3
4 |
picketfence
Harmless

Posts: 16
Registered: 15-1-2007
Member Is Offline
Mood: No Mood
|
|
Gas-phase apparatus construction
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
Has anyone ever constructed this?
The article details a safe gas-phase apparatus for the pyrolysis of acetone to ketene - which is not what I'm interested in particularly due to the
fact that if I needed acetic acid I'd just use vinegar and I have no requirement for the anhydride. Rather I'm wondering if there are any issues with
the actual construction of this apparatus.
Where would you get a combustion tube suitable? and the choice of glass isn't exactly perfect for my liking - quartz would be better/safer.
You could construct a quartz combustion tube with a quartz tube with a short section of narrower diameter quartz tube (as found in radiator heaters)
at each end for the inlet and outlet and adhere these with industrial high temperature adhesive.
In the diagram, what's 'B' called? Why is there a stopcock used above B, which collects the unreacted acetone?
Why is there a three-way stopcock used at the far right?
[Edited on 15-1-2007 by picketfence]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have never tried to make ketene but will take a crack at a couple of your questions.
The stopcock at "B" would be mostly closed as you collect acetone above it then open it periodically to drain the acetone. Or perhaps it could just
be "cracked" open to keep a liquid seal. If you just left it open you would blow out everything including the gaseous ketene. Edit: Actually that
valve looks more like a needle valve, which would allow fine adjustment for draining the acetone yet keeping the liquid seal.
I imagine the 3-way valve at the far right is to allow you to vent the product gas to the atmosphere at start-up until you're ready to start
absorption. Also it could serve as a vacuum breaker at shutdown to avoid any suckback.
What do you mean by:
| Quote: |
You could construct a quartz combustion tube with a quartz tube with a short section of narrower diameter quartz tube (as found in radiator heaters)
at each end for the inlet and outlet and adhere these with industrial high temperature adhesive.
|
[Edited on 16-1-2007 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Thermal
Harmless

Posts: 41
Registered: 31-1-2004
Member Is Offline
Mood: No Mood
|
|
IMHO setups using an electrically heated filament are much preferable.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The electrical ketene or butadiene lamps suffer from fairly rapid carbonization of the filament so that in practice to keep one running (their output
is SLOW) you need a spare filament assy to cgange out while cleaning the crudded up one.
The combustion tube approach is not very safe IMO if you are using open flame burners for obvious reasons but then safe and ketene are two words that
can't really be concatonated without destroying all epistomological value of the sentence.
A tube furnace of appropriate length and ID and temperature range is a better way to go. These are often available very cheaply on LabX (I was only
bidder on the auction in which I got mine, for the opening bid $70) and I agree that a quartz tube is a far better idea than pyrex for an operating
temperature c. 700 C. You might consider Vycor though which will be adequate and ought to cost way less than quatrz, be easier to fabricate
connection to and have superior mechanical qualities. The furnace I won for $70 accepts up to 2" OD tubes, is 12" end to end and has a top end of 1100
C.
[Edited on 16-1-2007 by Sauron]
[Edited on 17-1-2007 by Sauron]
[Edited on 17-1-2007 by Sauron]

|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Sauron you did indeed get a sweet deal.
I have been occaisionally checking out the tube furnaces on ebay, but they are either too expensive or don't get the temperature I want. It seems the
breakpoint is 1100-1200C and I'm looking for 1300C. I have a plan for a homemade tube furnace but it won't be cheap either. It would have a
clamshell design. It would use a 1648C castable Mizzou refractory in the center and a Kast-O-lite type material in the annulus (or a castable
insulating brick). The container would just be a 16 gallon steel drum. I'm thinking it would be about 18" long and have a bore of around 1.5". It
would use Kanthal A-1 elements operating at their maximum allowable temperature. It would have a programmable PID controller and TC for temperature
control.
Edit: Corrected refractory temp
[Edited on 17-1-2007 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
picketfence
Harmless

Posts: 16
Registered: 15-1-2007
Member Is Offline
Mood: No Mood
|
|
sauron you really did get a good deal, what's the inner diameter? It looks rather large! (too large?)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The ID is 2" (5 cm).
It's 1350 W and draws 11 A @ 110V. My mains are 220 so I put a rather larger stepdown in between that is rated for 3 KW so I don't expect any trouble.
[Edited on 17-1-2007 by Sauron]

|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Good price. Those are usually about 3,000 USD for the ones that get to 1200C. I use two such pieces of equipment in the laboratory I work at. One is
used for prepping catalyst, other for pyrolysis.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I made Ketene a few hours ago by the tube furnace method.
It worked surprisingly well, I didnt use any packing in the quartz tube, just an empty tube at 700°C and acetone vapor.
The ketene was lead into water in order to hydrolyze it to acetic acid and prove the succes of the method.
Here's the acetone evaporator:

The exit end of the furnace, with the excess length of the tube serving as cooling zone (too long, but still worked):

The condensation apparatus to condense unreacted acetone: the vapors are fed into a RBF with a dimroth condenser, the acetone collects in the RBF and
the ketene is withdrawn from the top of the condenser:

700°C in the furnace, a dull red glow, the digicam makes it look violet due to its sensitivity to infrared which is emitted in large amounts:

Upon starting to evaporate acetone, a white fog started to appear in the apparatus, and a brisk gas stream is noticed in the washing bottle:

20ml acetone were fed through the furnace, of which 7ml were recovered unchanged in the RBF (less than should be (60-80%), so the speed of
distillation was probably somewhat too slow).
The water in the washing bottle definately became acidic! There was some gas evolution when some CaCO3 was added to it, therefore proving that ketene
was being generated in this setup.
No soot or coke formation occured inside the tube! The reaction was very clean.
The exit gases from the washing bottle (methane, carbon monoxide, ethylene) were very flammable and burned well.
I carefully wafted the exit gases in order to find out how ketene smelled like. BIG MISTAKE! There was so much unhydrolyzed ketene present that I
choked and coughed, it felt like chloropicrin minus the eye irritation. Ketene is a really obnoxious lung and respiratory tract irritant. I also had a
stinging sensation in the chest for some time afterwards. Lets hope no delayed toxic effects will follow.
The Ketene also had a very unique and strong acrid smell.
In order to hydrolyze ketene, a washing bottle with sintered glass gas bubbler would have probably been better than a simple washing bottle.
Before any synthesis can be done with the ketene generated by this setup, the corks will have to be replaced by something more gastight.
[Edited on 12-8-2007 by garage chemist]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very nice GC! We have been talking about making ketene for years but this is the first time someone has actually done it, IIRC.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yes, it seems like I am the first to make ketene at home and document it. I saw some pictures of a homebuilt ketene lamp in another thread, but it
didnt appear to have worked.
The tube furnace method is without a doubt more suited to obtaining useful amounts of ketene than the lamp, and can be run nonstop for 24 hours or
more should the need arise (e.g. when gassing acetic acid) without having to change filaments.
I'll try to make acetanilide with the ketene when the teflon endpieces for the quartz pipe are ready. I'd like to use another solvent than ether,
since most of the ether will probably be evaporated out of the washing bottle due to the stream of inert gases.
Acetone springs to mind, since ketene has a high solubility in it.
Ethyl acetate would also be a good choice. But both also dissolve acetanilide well, making some sort of workup necessary before the acetanilide can be
weighed and yields calculated.
[Edited on 12-8-2007 by garage chemist]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Bravo! Now I'm interested, I'd like to give it a shot as well. A clean reaction eh?
I still need to finish up the SO3 work though :-\
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
....."Acetone springs to mind, since ketene has a high solubility in it. "..........
Wouldn't this react to isopropenyl acetate.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Obviously not, otherwise the ketene would react with the unreacted acetone (about half of the used amount), either in the hot or in the cold zone or
in the RBF...
EDIT: I looked it up. Isopropenyl acetate is actually made from ketene and acetone, but a strong acid catalyst is required.
Its absence in my setup is surely the reason why the ketene does not react with the acetone.
[Edited on 13-8-2007 by garage chemist]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by bio2
....."Acetone springs to mind, since ketene has a high solubility in it. "..........
Wouldn't this react to isopropenyl acetate. |
Takes a catalyst to get much yield, H2SO4 is often used. In cold acetone ketene dimerizes and also forms dehydroacetic acid (
3-Acetyl-6-methyl-2H-pyran-
2,4(3H)-dione, 3-acetyl-6-methylpyran-2,4-dione), SMILES O=C1OC(=CC(=O)C1C(=O)C)C as well as isopropenyl acetate, acetylacetone, and others.
Acetyleacetone is also a byproduct of making isopropenyl acetate.
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
So, is there any way to stabilize the ketene in a solvent
for short term storage?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
SFAIK it reacts with itself within a few hours, it's pretty much a make-it-use-it reagent.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
On Orgsyn they said it can be stored up to two weeks when liquefied at -80°C:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5...
It might be stored somewhat longer at liquid nitrogen temperatures.
Also, only the pure ketene gas obtained by cracking diketene is suitable for liquification, the gas prepared from acetone is of course saturated with
acetone vapor along with all the other gases it contains.
Ketene has high solubility in acetone (thats the reason why the condensed unreacted acetone should be kept close to its boiling point in the RBF, to
minimize ketene loss by dissolution), but it dimerizes completely to diketene overnight, which is a way to prepare diketene in the lab:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
Diketene itself is only stable up to a week, dimerizing a second time to dehydroacetic acid and becoming an orange to brown tar.
Ketene is, just like ozone, a reagent that is best made as needed. Once the apparatus (ozonisator + high voltage source for ozone, and pyrolysis setup
or "lamp" for ketene) is made, it is very easy to create a constant stream of the gas.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I had given up the idea of making it- now I'm reconsidering .. what is the furnace you're using?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Its a simple selfbuilt furnace, Ytong (expanded concrete) as the body, covered with refractory mortar on the inside and with 80/20 NiCr 1mm wire as
the heating wire in four spirals. 2000 Watt at 230V. Power is regulated by a thyristor circuit.
The furnace did cost maybe 20€ to make, not counting the thyristor circuit and the quartz tube.
Pyrex would probably still be usable at that temperature, but you'd have to be careful to avoid big temperature gradients.
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
Touche
Awhile back me and Fleaker decided to try garage chemist's acetone pyrolysis to ketene. It worked pretty well. We used one of his swagelock stainless
steel reaction tubes and packed it with really fine copper wool. We then filled a RBF with acetone and put it in a mantle and hooked it all up to the
reactor tube and set it to bake at 700 celsius. On the product side we set up dual cold water condensors set up reflux style to collect any acetone.
The ketene went through and into a gas washing bottle filled with some glacial acetic acid. All of that was in a big beaker filled with ice, which
helps the ketene absorb better and saturate the acetic and react. All of the leftover ketene went into a second wash bottle filled with water. Fleaker
titrated the slightly acidic solution and found out that it was 0.330M and this was from the second wash bottle. All this tells us was how much
ketene escaped to hydrolyze in the second bottle. pH was 3.8 on that solution.
There was a lot of gas produced, and it moved quickly through the system like a gray cloud. This of course was out ketene, ethylene, and carbon
monoxide which are byproducts of the pyrolysis and degradation of the ketene.
We used about 250mL of acetone, collected about 100mL of it, used 200mL of glacial acetic acid in the wash bottle, and after fractional distillation
(which went shitty, but got 3 fractions) and freezing out the GAA, we got about 62mL of Ac2O. Not bad for 3 hours work, and only an hour of that was
spent pyrolyzing, most was spent setting up, titrating, and yelling at Fleaker for almost killing us.
To confirm that we were making some ketene, we took some gas samples from the second bubbler trap and did some FTIR on it and saw that we got some
carbonyl in the spectrum. I don't have a scanner here, but if enough of you want the spectrum I can go scan it in. We had a huge peak of carbonyl
at~1750 cm-1, some ethylene bands at 980/1390/1880 or so, big methane at 1380 and 2980ish and a nice big 2130 for CO. 980 was the big absorption band
for ethylene, the others were minor. I think the carbonyl one is probably ketene, I don't see acetone making it through two condensors and two
bubblers.
I took it home, put it all in the freezer and nothing froze. I figured there's probably a shit ton of acetone and GAA in there. So I did a quick
distillation.
After the fractional distillation, I put all the three fractions in the freezer. The first fraction, 30mL mostly acetone (came over at 34-84*C), next
140mL was GAA at 80-100 , then next was GAA and ~100ml GAA/Ac2O mix at 100-102. And I know that its not right--wonder about azeotropes...don't think
they exist for this system??
Put all three fractions in the fridge, only the GAA middle fraction froze. Put the other two in the freezer, and the first fraction didn't freeze,
took the last fraction a full day to finally freeze. Opened it up and woosh smells like Ac2O. Poured the liquid that didn't freeze and it measured it
in a grad cylinder. Probably a few more mls in there stuck in the GAA crystals.
Problems with this reaction included controlling the flow rate, it seemed like about 2/5 of the acetone we put in collected in the air trap. I think
the ketene should have had a longer residence time in the glacial to react more. Oh, and probably the biggest mistake was when Fleaker forgot to
tighten the apparatus and we had a small fire. Dumbass.
To improve this and make it more of a continuous process I'd say you should use a large equal pressure addition funnel filled with acetone on the
input side of things.
Conclusion:
Ac2O from acetone is easy and cheap, and not bad yield wise if you run it all day long.
So you all probably want pictures. We have some.

This is the input side. RBF with mantle.

This is the acetone & copper. (ya its sideways get over it)
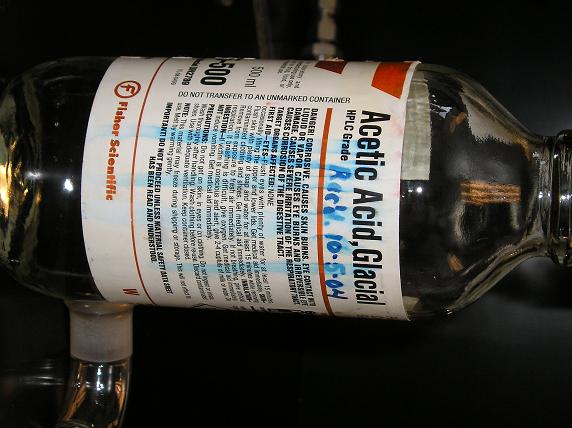
This is the GAA.



This is the output side with RBF and condensors and the traps. Note the big beaker with ice, that's where the bubbler with the GAA went.

A shot of the titration.

Pictures of the product.

Picture of the Ac2O and frozen GAA.
Hope you all liked it.
[Edited on 22-9-2002 by NERV]
[Edited on 22-9-2002 by NERV]
Vir sapit qui pauca loquitur.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Very nice, looks good! I like it!
Tube furnaces are cool, arent they? Unfortunately mine broke, I have to build a new one.
There are no azeotropes in the GAA/Ac2O system, but wou will want to use a column next time to get better separation.
You should test your product for presence of Ac2O by reaction with e.g. aniline in aqueous solution and see if it makes any acetanilide (Ac2O reacts
with aniline immediately at room temp). Acetanilide would precipitate.
Did you try to smell the ketene?
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Yes we wafted it and it does not smell very good. Good idea on the acetanilide. That is very easy to do.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Tube furnace insulation: Fiber or Brick?
Now that the ceramic tube has been ordered, the tube furnace project continues.
I am still undecided if I should use either ceramic fiber mats or lightweight firebrick as the insulation. Money is a minor factor, none of both
offers a significant lower price than the other.
Some considerations were given in this thread:
https://sciencemadness.org/talk/viewthread.php?tid=9412
and I wish to continue the discussion here since this thread is more appropriate topic-wise.
If I use fiber mats, I would get a 25mm 1400°C fiber mat of the dimensions 820mm*610mm (the unit the shops sells the mat in).
The tube of the furnace is 50cm long and 4cm in diameter, but the outer diameter is increased by the heating wire and electric insulation paste on it,
so I'll assume it to be 5cm in diameter.
If I apply two layers of the fiber mat, for an insulation thickness of 5cm, the mat piece I gave above would suffice (after cutting to size). If I had
to use an additional layer, I would have to get more of the fiber mat.
Is 5cm fiber insulation enough, or would 7,5cm be more appropriate?
I could also get the same dimension of fiber mat, but with 1250°C classification temperature, for somewhat less money, so I could make the insulation
thicker. Now that I think of it, this would probably be just as good as the 1400°C mat for my application. I take it that the fiber mats dont fail
immediately when their rated temperature is exceeded for a short period of time?
With firebrick, there are two types I can get: both 250x124x64mm in dimension, but one for 1150°C and the other one for 1400°C. Also, the former
weighs 0,9kg but the latter 2,2kg!
I would use four of those bricks (with a groove in them for the tube), giving a minimum insulation thickness of about 3cm. Sounds somewhat thin, but I
could use cheap rockwool or glass wool as additional insulation if the outside of the furnace gets too hot.
What happens to lightweight firebricks if their temperature rating is exceeded slightly, say if the 1150°C type is used at 1250°C for a short period
of time? Will they rapidly fail, or will they just develop some small cracks on the hot side?
And the quite heavy 1400°C ones, do they insulate less good than the 1150°C ones? Is 3cm of insulation too thin?
I'm leaning towards the fiber mat, as the construction of the furnace would be incredibly simple with it, and the furnace would be very lightweight
and transportable. But I need to know how thick the insulation should be.
With the firebricks the furnace would be much more rugged and mechanically stable.
What would you use for a tube furnace if you had the choice between kaowool and kiln bricks?
[Edited on 8-11-2007 by garage chemist]
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
You could just wrap your heating element wire around the tube, then put it in a box or pipe with heat resistant end plates and fill the whole thing
with diatomaceous earth for insulation. I found 25 lb boxes of snow white DE filter material at Home Depot for $18 each in the pool supply section.
Very low density material. It's supposed to be fairly pure silica, so the upper temperature limit should be as good or better than thermal blanket
fiber.
[Edited on 11-8-2007 by Eclectic]
|
|
|
| Pages:
1
2
3
4 |