soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
molecular sieve and acids (esterification)
I'm about to run an esterification reaction using n-acetyl cysteine, ethanol, and sulfuric acid (catalyst).
I want to run it with molecular sieve (3A) to take off the water produced by the reaction.
I'm wondering if the sieve will have problems with the sulfuric?
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
You might be interested in this preparation posted by nurdrage.
https://www.youtube.com/watch?v=Ah5ds_3s5BI
Quite a clever idea and a good way of increasing yield.
I am yet to try it myself.
|
|
|
soma
Hazard to Others
  
Posts: 297
Registered: 31-7-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for that link.
I'm wondering how necessary the sulfuric would be if the water is removed by molecular sieves. I think in the reaction using n-acetylcysteine, higher
temps would cause dimerization (it's run under nitrogen at room temp) http://www.sumobrain.com/patents/wipo/Method-preparation-n-a...
Also, I'm trying to find out if there's a 3A molecular sieve that isn't sensitive to strong acids. There are some sieves that are not acid sensitive.
[Edited on 4-6-2017 by soma]
[Edited on 4-6-2017 by soma]
|
|
|
j_sum1
Administrator
       
Posts: 6229
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I recall asking the same question once. The role of the concentrated sulfuric acid is catalytic -- it is not just there to mop up the H2O produced.
So it is still needed.
I don't know about non acid-sensitive molecular sieves. My understanding is that zeolites are damaged by strong concentrated mineral acids. But I am
definitely not the best person to ask.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Effective way for synthesis of Ethyl ester of carboxylic acids
Quote: Originally posted by soma  | I'm about to run an esterification reaction using n-acetyl cysteine, ethanol, and sulfuric acid (catalyst).
I want to run it with molecular sieve (3A) to take off the water produced by the reaction.
I'm wondering if the sieve will have problems with the sulfuric? |
There is no need for adding Molecular sieve in the mixture.Use below schema instead.
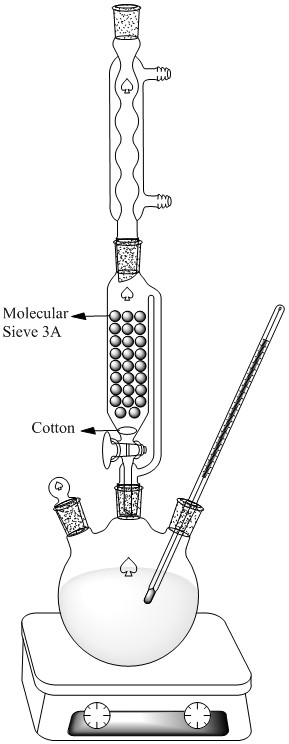
Formed water make azeotrope with EtOH and Molecular sieve will absorb it.This push equilibrium to Ester side.
[Edited on 4-6-2017 by Waffles SS]
|
|
|
tshirtdr1
Harmless

Posts: 31
Registered: 12-12-2014
Member Is Offline
Mood: No Mood
|
|
If you heat the reaction flask above the boiling point of water, the sieves won't do you much good. Also, they are expensive and just going to muck up
your reaction. If you must use sieves, do it as described above. I have done that and it works well. I have done esterifications over dry silica beads
and that works well. Again, you have to keep it below the boiling point of water. Of course, if you are using ethanol, then you shouldn't have a
problem with that.
[Edited on 10-6-2017 by tshirtdr1]
|
|
|
alking
Hazard to Others
  
Posts: 252
Registered: 11-3-2016
Member Is Offline
Mood: No Mood
|
|
Are you sure you have to keep it below the bp of water? Sieves are pretty good at holding on to water and it takes considerable heat and time to fully
dry them.
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
what about dry mgso4? It doesn't react with the sulfuric and is an excellent drying agent + it doesn't dissolve much in ethanol.
|
|
|