wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Small UVC lamp for in flask use
My small UVC lamp finally arrived today.. It’s small enough to fit thru a 24/40 joint for in flask use. Its only 3W input power but as it will be
used in flask I am hoping that will be sufficient that chlorination do not take days.
As is common for listing in English from China suppliers the spec is confusing:
Specification:
Material: quartz glass
Type: air sterilizer germicidal purifier lamp
Feature: non-ozone, unscented, UV light
UV size: 253.7nm
Light failure(%): 10%-15%
Adaptable power: 3W/5V
http://www.ebay.co.uk/itm/UV-Light-Air-Purifier-Quartz-Glass...
It was only £1.97 so I will try it. I will mount it on thermometer adapter with part of the stem cut off and figure out how to power it

[Edited on 7-8-2017 by wg48]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
What are you trying to chlorinate? I was able to chlorinate toluene with just visible light very quickly and very selectively using methods I posted
here:
http://www.sciencemadness.org/talk/viewthread.php?tid=14063
Basically, add a catalytic amount of bromine, and radicals will form throughout the volume of the solution from slightly longer wavelengths of light,
rather than just in the first few mm.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
j_sum1
Administrator
       
Posts: 6221
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
This looks like a cool concept. Economical and tidy.
It would need to be effective of course and Melgar's procedure is a good reference point for determining that.
Let us know how you go.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
wg48,
If you put your uv lamp in a Pyrex thermometer well you will lose most of your light energy below ca 300 nm. Pyrex is an excellent uv filter for short
wavelengths. You will need to use a quartz immersion well to transmit below 300 nm. Moreover, 3w is a pretty small energy level to do any serious
photochemistry. As MEegar found, ordinary sunlight is quite satisfactory for halogenations.
see Calvert and Pitts, "Photochemistry, " Wiley (1966), pp748-749.
AvB
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
http://www.ebay.com/itm/3W-High-Power-UV-ultraviolet-365nm-3...
3w 365-370nm UV LED for $1.30 US free shipping. Pretty effective cost wise and small enough to fit places tight. I bought two a while ago,
soldered them up and had a headache for the rest of the day,. = they work lol.
I have not tried them for any chem fun, but I will say they look a lot different than most UV led's. A lot more white, no carry over purple. Thought
I had been had, but my rock specimines glowed without fail. If one could find a quartz plate to put over a joint opening, it could be shined in.
Other materials may be useful here, just brainstorming
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  | What are you trying to chlorinate? I was able to chlorinate toluene with just visible light very quickly and very selectively using methods I posted
here:
http://www.sciencemadness.org/talk/viewthread.php?tid=14063
Basically, add a catalytic amount of bromine, and radicals will form throughout the volume of the solution from slightly longer wavelengths of light,
rather than just in the first few mm. |
The side chain of toluene was at the top of my list. Your simple method appears to have made mine redundant.
I thought a UVC low-pressure mercury lamp was required to spit chlorine judging from what I had read. I thought UVC was required to match the UV
absorption of chlorine. At first I considered UV LEDs. But UVC LEDs are very expensive. So it had to be a UVC mercury lamp but cheap UVC mercury lamps
are too large to fit in flask to avoid the Pyrex absorption of UVC. When I spotted that lamp on ebay it seemed perfect..
Bromines peak absorption may be in UVB/A or violet range of cheap LEDs and not significantly absorbed by Pyrex so your method may be perfect. Just pop
a string of LEDs in to a thermometer adapter, no sealing problems and you get a lot more photon per watt because of the high efficiency of LEDs at
those lower frequencies.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by violet sin  | http://www.ebay.com/itm/3W-High-Power-UV-ultraviolet-365nm-3...
3w 365-370nm UV LED for $1.30 US free shipping. Pretty effective cost wise and small enough to fit places tight. I bought two a while ago,
soldered them up and had a headache for the rest of the day,. = they work lol.
I have not tried them for any chem fun, but I will say they look a lot different than most UV led's. A lot more white, no carry over purple. Thought
I had been had, but my rock specimines glowed without fail. If one could find a quartz plate to put over a joint opening, it could be shined in.
Other materials may be useful here, just brainstorming |
I have ordered two similar to those to play with. But they are not UVC which I thought was required.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AvBaeyer  | wg48,
If you put your uv lamp in a Pyrex thermometer well you will lose most of your light energy below ca 300 nm. Pyrex is an excellent uv filter for short
wavelengths. You will need to use a quartz immersion well to transmit below 300 nm. Moreover, 3w is a pretty small energy level to do any serious
photochemistry. As MEegar found, ordinary sunlight is quite satisfactory for halogenations.
see Calvert and Pitts, "Photochemistry, " Wiley (1966), pp748-749.
AvB |
As I said I will cut the end of the adapter off.
I thought it would be obvious I would then mount the lamp so it protrudes from the end and sealed. Sealing with chemical resistance remains a
problem.
The point of the "in flask" or immersion is to avoid the absorbtion of the pyrex of the flask which would be pointless if replaced by the absorbtion
of the thermometer adapter.
Yes 3W is very small. I was hoping as its effecently coupled to the reaction volume (in flask) it would still have a signicant effect.
Yes Melgar's may have solved it for chorination.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I know people who used to just set up their reaction in a Pyrex baking dish and sit on top of the roof all day. They got great yields. Works best
with non-volatile chemicals.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by wg48  | The side chain of toluene was at the top of my list. Your simple method appears to have made mine redundant.
I thought a UVC low-pressure mercury lamp was required to spit chlorine judging from what I had read. I thought UVC was required to match the UV
absorption of chlorine. At first I considered UV LEDs. But UVC LEDs are very expensive. So it had to be a UVC mercury lamp but cheap UVC mercury lamps
are too large to fit in flask to avoid the Pyrex absorption of UVC. When I spotted that lamp on ebay it seemed perfect.. |
My working theory on why bromine catalyzes this reaction is that it forms BrCl, bromine monochloride. This will absorb longer wavelengths than just
chlorine by itself, and when it does so, creates a bromine radical and a chlorine radical. So you're still creating chlorine radicals that are just
as active as they'd be from any other reaction, regardless of what the bromine radicals are doing.
| Quote: | | Bromines peak absorption may be in UVB/A or violet range of cheap LEDs and not significantly absorbed by Pyrex so your method may be perfect. Just pop
a string of LEDs in to a thermometer adapter, no sealing problems and you get a lot more photon per watt because of the high efficiency of LEDs at
those lower frequencies. |
I've successfully used T12 fluorescent tubes, white LEDs, high-pressure sodium, and sunlight to initiate this reaction. It's actually a chain
reaction, and you need a high concentration of chlorine to propagate it. But with a high chlorine concentration, all the UV quickly gets absorbed,
and radicals only form within the first few millimeters of the surface. With bromine monochloride, the molecules are scattered throughout the
solution and absorb light that would have previously been transmitted, forming radicals.
Of course, that's only a hypothesis, but it does seem to explain it.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I found the absorption spectrum for Cl2(g) and as a bonus BrCl and Br2.
If I am interpreting the Cl2 data correctly the dissociation peak is at 330nm with the 50% of peak values at about 305nm and 365nm. That is not UVC
its between UVA and UVB, just on the UV absorption edge of borosilicate 3.3 glass and in range of cheap 365nm UV LEDs and borosilicate glass.
The BrCL peak is at 370nm with 50% points at 345nm and 415nm
The Br2 peak is at 420nm with 50% points at 385nm and 495nm (peak in the violet part of visible spectrum).
The CL, BrCL and Br2 peaks are in the ratio of 2:3:5 appr.
Note: if Melgar's synthesis requires spliting Br2 it does appear to only need the violet of a visible light source for example a violet LED.
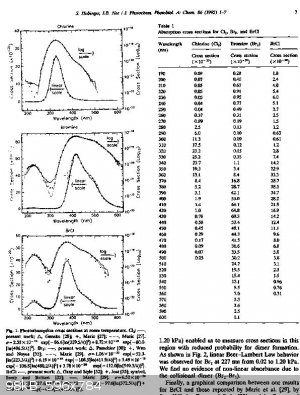
[Edited on 8-8-2017 by wg48]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I'd actually posit that what's more important than the peaks and the 50% values, is the maximum wavelength that can cause a disassociation. The
reason is that these are chain reactions, and you don't want all the light to get absorbed immediately. You want it to penetrate into the liquid some
ways before being absorbed. After all, if you have too many radicals generated in too small of a volume, the radicals just end up reacting with each
other again. They need to be more dispersed, so that they're much more likely to react with your desired substrate than each other.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
j_sum1
Administrator
       
Posts: 6221
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by Melgar  | | I'd actually posit that what's more important than the peaks and the 50% values, is the maximum wavelength that can cause a disassociation. The
reason is that these are chain reactions, and you don't want all the light to get absorbed immediately. You want it to penetrate into the liquid some
ways before being absorbed. After all, if you have too many radicals generated in too small of a volume, the radicals just end up reacting with each
other again. They need to be more dispersed, so that they're much more likely to react with your desired substrate than each other.
|
Would stirring help or is the reaction process too rapid for that.
I ask because I know little about radical chemistry.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
You are sparging with gaseous chlorine, correct? If so, this will provide stirring.
[Edited on 9-8-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
j_sum1
Administrator
       
Posts: 6221
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Makes sense. 
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Industrially, when doing free-radical chlorination, they do stir very rapidly. The bromine seems to greatly reduce the need for stirring though,
because there isn't the need to quickly move the formed radicals away from the surface of the flask.
I actually did a lot of research back when I discovered this, and the only reference I found to using bromine for free-radical chlorination was in
Ullmann, in the section on benzotrichloride, there was a note that adding bromine could speed up the reaction time. This was after I'd already done
the reaction and posted it here though.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
So I tried to light up my miniature mercury lamp. It needs a round 1kV to strike judging by the position of a variac driving a ballasted 2.2kV
transformer . I was hoping to power it from an inverter of a compact florescent light bulb suitably modified to drive the lamp at 3W but I don’t
think one of those will output 1kV in start up mode.
I was only able to run it at an estimated input power of 1W (limited by the HT transformer hacked from a bug zapper). No smell of ozone and it did not
make anything glow that normally glows with a black light. Small blobs of what I assume is mercury is visible in the tube and the glow looks like a
mercury vapour discharge but I have a suspicious nature. So I tried a piece of the glass envelope from broken florescent light bulb that still had the
phosphor on one side . When the phosphor is directly exposed to the lamp it does glow but not when exposed thru the glass (the lead glass absorbs the
UV.). The phosphor does not glow with a black light. I also tried Borosilicate 3.3 and what I believed to be soda glass both as expected blocked the
glow of the phosphor. A quartz tube did not.
As a comparison I tried a 130W high-pressure mercury lamp that had been removed from its outer secondary envelope. It was powered by the same HT
transformer so it was running at about the same current as the miniature lamp and probably at a similar power. Within a few seconds of it turning on I
could smell ozone. It too excited the phosphor directly and thru quartz but not thru boro or soda glass.
So my miniature lamp probably has a doped quartz envelope that absorbs the wavelengths that create ozone. The spec is correct it stated “no
smell”. Apparently the short wavelengths are not needed for chlorination but I would have preferred to have that option.
I noticed an odd effect. When the connection to the miniature lamp was loose and arcing the colour of the discharge changed from light greenish blue
to almost white and brighter. . I suspect the output capacitance of the transformer was being charged to a higher voltage and then discharging in a
short high current pulse.
On a related subject: I have often wanted to check that the glassware I had purchased really was borosilicate. If the emission of 365-370nm or perhaps
370-375nm UV led is between the UV cut-off frequency of borosilicate and the longer wavelength UV cut-off frequency of soda glass and a suitable
phosphor is available then they may provide a simple method of confirming a glass item really is borosilicate glass.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by violet sin  | http://www.ebay.com/itm/3W-High-Power-UV-ultraviolet-365nm-3...
3w 365-370nm UV LED for $1.30 US free shipping. Pretty effective cost wise and small enough to fit places tight. I bought two a while ago,
soldered them up and had a headache for the rest of the day,. = they work lol.
I have not tried them for any chem fun, but I will say they look a lot different than most UV led's. A lot more white, no carry over purple. Thought
I had been had, but my rock specimines glowed without fail. If one could find a quartz plate to put over a joint opening, it could be shined in.
Other materials may be useful here, just brainstorming |
Violetsin:
My 365-370nm UV LEDs arrived yesterday. I have now tried one. I too think I may have been had. It does not make phosphors from inside a florescent
tube glow but it does make things glow that glow with a black light and it can do so thru black light glass, soda glass, lead glass (light bulb
glass), Pyrex 3.3 and quartz.
Could you determine if your LED fluoresces the phosphor from inside a florescent light tube, does still makes your rock specimens glow thru soda glass
and pyrex? Does it make white cotton or paper glow?
|
|
|