metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Na2CO3 + charcoal makes Sodium revisited
Seven years ago I tried to make Na and K metal by heating their carbonates with powdered charcoal to 1200 C in a steel retort. As the vapors which
appeared, there was indeed alkali metal formed, but it burned again, so the proof of concept succeeded.
https://www.youtube.com/watch?v=9l_DojAugyg
https://www.youtube.com/watch?v=nUQNjO32vyQ
But now I have ordered Ar gas to flush the retort, make a stainless steel retort and moderate the heat with a sandbox to get a more even heat
distribution over the bottom of the retort to prevent overheating. In earlier experiments, the (mild steel) retort melted locally at the bottom due to
overheating and moreover, the alkali metal burned due to traces of air.
Here a sketch how I want to make the retort. Does somebody have better ideas ?
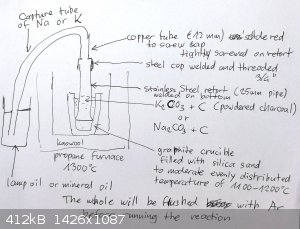
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
What exactly is the reaction that is taking place? Wouldn't CO2 form somewhere and react with the Sodium?
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It produces CO. See: https://www.sciencemadness.org/whisper/viewthread.php?tid=76...
The net reaction would be:
Na2CO3 (s) + 2 C (s) -> Na2 (g) + 3 CO (g)
although I would suggest using a large excess of carbon.
You are correct that CO2 would react with the gaseous sodium.
[Edited on 8-30-2017 by Metacelsus]
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Why carbon? Just for the challenge?
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Indeed for the challenge, but in this way the K is distilled. This used to be a commercial process in the late 1800s. I tried electrolysis but that is
a far more tedious process. I will flush the retort with Ar gas before running.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I read about old potassium making processes some time ago, and I think I recall there being, for that process, some black byproduct that would build
up in the condenser(nose of the retort?) which was a potential hazard(explosive?).
I may be confusing two processes here, or just be plain wrong. I'll see if I can find the description I read.
This was some old source material, so they obviously weren't doing the process under an argon atmosphere.
I think they were condensing it into 'rock oil', or into flat containers of some sort immersed in 'rock oil'.
And It's possible it was just some byproduct that clogged the nose of the retort and wasn't hazardous in and of itself.
Will post again if I find anything more definite than my hazy memories..
@halogen,
thanks for filling in the blanks, because I couldn't find that old reference, but had an uneasy feeling about it.
[Edited on 9-9-2017 by SWIM]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
The hazard of the process was indeed that black byproduct. Possibly only potassium intercalcated graphite, possibly funnier things. Research, in
modern times, on the reduction of carbon monoxide by alkali metals in solution reveals such products as acetylenediolate, and deltate. The intimate
mixture necessary for reasonable yields of potassium was ensured by the combustion of tartrate salt, a byproduct of vinous fermentation. Other carbon
bearing salts worked.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Mermaidkiller is metalresearcher on here :O working on challenging projects
Potassium bitartrate is cream of tarter in stores
Kind of expensive given the amount
The black deposits sounds like pyrophoric potassium graphite
Or potassium carbide both doesnt seem possible in the condensing phase more likely finally divided potassium metal
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I tried it a few times, but I probably added too much charcoal.
I heated it 10-20 minutes to 1200 C in a small propane furnace in a steel retort flushed by Ar gas.
I used powdered charcoal.
After running the test (a few grams, 4:1 weight ratio K2CO3 / C, which is well excess of C to prevent oxidizing) and opened the retort after allowing
it to cool, some black crud appeared which caught fire in air but not with the familiar magenta K color, but just a 'fire' solor.
Dumping it in water resulted in CaC2 like reactions with bright very sooting flames.
Is this K2C2 ?
So next run will be ratio 6:1 which is stoichiometrical.
K2CO3 + 2 C => 2 K + 3 CO
140 24
140:24 is about 6:1.
[Edited on 2017-9-19 by metalresearcher]
|
|
|