sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
ammonia generator
https://phys.org/news/2014-08-air-ammoniaone-world-important...
https://www.ncbi.nlm.nih.gov/pubmed/25104378
You can you give an opinion about this method?
[Edited on 24-9-2017 by sclarenonz]
[Edited on 24-9-2017 by sclarenonz]
Attachment: AMMONIA GENERATOR.rtf (37kB)
This file has been downloaded 649 times
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's hard to do efficiently on an amateur scale... if you just want some ammonia gas, you can boil a concentrated solution of ammonia and dry it by
passing it through a tube of sodium hydroxide or perhaps calcium oxide.
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
THANK
THANK YOU, BUT I'M STUDDING A STRONG AND SUSTAINABLE FUEL AMMONIA WAS THE GREAT CHOICE FOR BEING REMOVED FROM THE AIR, I THINK THAT A SMALL AMMONIA
GENERATOR IS NOT DIFFICULT, IF YOU CAN READ THIS ITEM AND TELL ME WHAT DO YOU THINK?
"In the absence of the nano-Fe2O3, water is simply electrolyzed into hydrogen at the cathode and oxygen at the anode in the 200°C molten hydroxide
chamber. In the presence of nano-Fe2O3, two alternative mechanisms of the ammonia synthesis can be considered. In the first, electrochemical reduction
of water to hydrogen occurs at the cathode, which then diffuses to react with adsorbed nitrogen on the nano-Fe2O3 surface to form ammonia. "
"During the last 2 hours of a 200°C (NaOH-KOH) 6-hour, 2 mA cm−2 run, the ammonia production rate fell to 85% of its average value over the first
4 hours"
[Edited on 24-9-2017 by sclarenonz]
[Edited on 24-9-2017 by sclarenonz]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
We already produce millions of tons of ammonia a year, via the Haber-Bosch process. They currently use hydrogen made from steam and natural gas, but
hydrogen could be used from, say, the sulfur-iodine cycle (uses thermal energy directly to generate hydrogen) and the hybrid sulfur cycle can both be
used to generate hydrogen and oxygen using primarily thermal energy:
https://en.wikipedia.org/wiki/Sulfur%E2%80%93iodine_cycle
https://en.wikipedia.org/wiki/Hybrid_sulfur_cycle
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
thank
nteresting, did not know about this process, thank you !!
I'm looking for something simple and can be done at home, I know that the enzyme nitrogenase(https://en.wikipedia.org/wiki/Nitrogenase) does this process inside the water, the water itself cools, I put ne a great all of iron, and I put 2
wire strands that I know are made of nickel and steel because they have a lot of malleability, I put my compressor to throw the air that has 70% of
nitrogen the air will react with the hydrogen soon after the electrolysis, I joke small pieces of aluminum, that with a solution of 5 mol of naoh and
with electricity will generate much hydrogen, the hydrogen will get stuck in the molecule of iron oxide and the nitrogen will get stuck in the same
way.WE HAVE TO IMITATE NATURE!!, what do you think about it, I will withdraw after 6 hours of reaction my tube is 1 meter high, I will still see if it
will work, this is only theory if someone can help me,
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Nature is actually much worse at fixing nitrogen than humans are. Roughly half of the fixed nitrogen in the ecosystem was fixed by humans, not
nature. If you insist on fixing nitrogen in a way that nature does it though, you can generate nitrogen oxide species using electrical sparks. This
happens during lightning storms in nature, after all. It is not efficient at all, though. The Haber-Bosch process is MUCH more efficient, but it
requires very high temperatures and pressures, and you will not be able to do it at home.
Do you know how important it was for militaries to obtain fixed nitrogen? VERY IMPORTANT! France and England got their fixed nitrogen by controlling
access to islands where seabirds nested. They would send boats there and mine these islands, which were covered by thousands of years of accumulated
seabird poop. They would then extract the ammonia and nitrates from the seabird poop and use it to make explosives. If it were easy to generate
ammonia from nitrogen at home, then someone would have figured out how to do it long before the Haber process was discovered. When Germany finally
discovered the Haber process, it allowed them to produced enough explosives to fight wars against France and England, and Germany immediately fought
two very large and very well-known wars, against most of the rest of the world. Germany had all the best chemists in the world for most of the 19th
century, but was unable to fix atmospheric nitrogen until the 20th century, so don't be surprised if you aren't able to figure out a workable process
by yourself with just your own resources.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
thanks, yes this is true, but there is another method that looks better:
https://en.wikipedia.org/wiki/Calcium_nitride
https://en.wikipedia.org/wiki/Sodium_nitrite
What do you think about calcium nitride?
my doubt and I know that there is an exit is about a great independence for my life I want a sustainable life and learn to recycle everything I get
from nature, in the great proportions you are right, the process haber bosh wins, but in small proportions nature wins , because at the microscob
level there are huge amounts of ammonia, imagine if I increased this technology from nature to a larger scale, that's the way I'm thinking, I know
there are alternatives that can be done with nothing, and with each passing day old ideas They are being abandoned, it is difficult to accept, but
everything is possible.
I'm going to try and wonder how to identify small amounts of ammonia?
[Edited on 29-9-2017 by sclarenonz]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Okay, if that's what you want, then use lithium nitride instead:
https://en.wikipedia.org/wiki/Lithium_nitride
I made the mistake of heating lithium in an atmosphere that I THOUGHT it was inert to once, and after the ensuing small red fire, I learned of this
fun compound. The bonus of lithium nitride is that lithium metal is much easier to prepare from its salts than calcium is, which facilitates
recycling the lithium in a closed-loop process. Lithium nitride will also react with any protic solvent to produce the solvent's lithium salt and
ammonia.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
Thank you, very interesting, but I was thinking I spend a lot of time and energy, sorry for the simple questions but I am an amateur who is already
tired and desperate to leave the local commerce and his financial system, was testing the results with Turmeric told me that it is great for dedecting
ammonia is a deep red, tested and turned quite red, but I need to test with other chemicals to see if it turns red. thank my brother
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
The easiest test for ammonia is to add a hydroxide salt and smell it. You can smell ammonia quite well even at low concentrations.
You could always get ammonia the way people used to during the middle ages, and leave a bunch of animal scraps mixed with urine in a closed barrel.
Or just urine. It would be easier. If you just want fixed nitrogen for fertilizer, then you can grow legumes (soy, alfalfa, peanuts, etc.) on the
land, and rotate crops so that the soil doesn't get depleted.
But really, fertilizers like synthetic urea are so cheap, that they will pay for themselves with the increase in yield. It is the benefit and the
curse of having a market economy. My dad tried to do what you're considering doing, and tried to do everything the hard way and be self-sufficient,
but then what do you do if you injure yourself and get an infection? You have to go to the doctor. And what about when you get cavities in your
teeth? Or your equipment breaks? Or some idiot steals your stuff? You need money for all those things, and often considerable amounts of money.
[Edited on 10/2/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I read your post a few minutes ago and I was about to recommend HCl gas (vapours from an open bottle) to detect ammonia gas
- white ammonium chloride 'smoke' forms,
but I thought I'd have a go with turmeric ... FANTASTIC !
just a hint of ammonia solution turned a whole test tube of turmeric suspension from yellow/orange to deep red.
A very sensitive indicator.
Turmeric is now a part of my chemistry kit,
and I feel a curcumin (or whatever is the ammonia sensitive compound) extraction is comming soon.
I may also make some turmeric paper indicator strips.
Does anyone know if other chemicals have a similar effect on turmeric that may give false (or equally useful) indications ?
P.S. I read that curcumin changes colour if Boron ions are present, but neither borax nor boric acid gave a significant colour change 
[Edited on 2-10-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Another use of the smoke effect with just two drops of ammonia makes for a good illustration towards the end of this clip.
https://www.youtube.com/watch?v=95mCNQnyiBk#t=1m29s
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
thank you for the great clarification, today it is impossible to live without the money, but I have many friends who went to live in the Amazon and
they get everything they want from nature, I remember that my school had no recourse and in the backyard of the school the teacher took the The root
of the plant: juglans regia and was a powerful acid-base indicator, but today everything is deforested and I look for this tree and can not find it,
how to make some time I go to the forest, live with the Indians and ask where there is this tree:
https://en.wikipedia.org/wiki/Juglans_regia
but I know it exists in nuts:
https://en.wikipedia.org/wiki/Aesculin
the red cabbage is very famous, but it is not specific as the turmeric
I really liked that smoke is enough to make kids fall in love with science, I'll try to do
there is a book that I found very cool, teaches to make potassium nitrate with ash and hot water, I found it very interesting
Attachment: improvised-munitions-handbook.pdf (4.9MB)
This file has been downloaded 343 times
[Edited on 2-10-2017 by sclarenonz]
|
|
|
Texium
|
Thread Moved
2-10-2017 at 06:35 |
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Which would be more effective for generating ammonia gas, heating ammonium sulfate or urea?
Also, if looking to cool the gas down before bubbling through water, how will hot ammonia gas effect copper tubing? I assume that it would make a
copper amine complex coating (deep blue color) but would most of the gas pass uncontaminated through into the water? I'm sure stainless tubing would
be better, but that is often more expensive I believe..?
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
Thank you for teaching me how to convert ammonia with hydroxide. This was extraordinary. I did not know. I remember that a very smart person wrote a
nitric acid teaching topic about urea or ammonia. He talked about a system very similar to you. is describing, I do not know where this topic is but I
saved the picture and you text:
Combustion of ammonia without a catalyst results in: 4NH3 + 3O2 ===> 2N2 + 6H2O, so that it would not work. With a platinum catalyst (some say
copper works well), the reaction will be: 4NH3 + 5O2 ===> 4NO + 6H2O. This is the industrial way of producing nitric acid and nitrates. However, in
a home environment, this requires very sophisticated equipment or very intelligent design. To give you an idea of how it is, I'll write the
rough configuration (which my limited intelligence can think of).
Firstly, you will obviously need the container to make pyrolysis of urea. A flat bottom balloon will work well. 2: A U-shaped glass tube to channel
the gases produced into an oxidation chamber with some loose copper catalyst. This chamber may most likely be a three-way flask, one opening being for
the gases, the other for placing an air pump to inject air, and the latter leads to another glass tube leading to a glass vase. Both the first and the
second balloon have to be heated. The final glass tube takes the gases into a vazo with cold H2O or H2O2, the gases will dissolve, and the air coming
from the fish pump goes away
Now, the products produced: Urea Pyrolysis: CO (NH2) 2 ===> NH3 + HCNO. HCNO hydrolyzes in water to produce NH 3 and CO 2, which is in water to
form NH 4 CO 3, which reacts with HNO 3 to form NH 4 NO 3. Therefore, its final product is a mixture of HNO 3 and NH 4 NO 3.
If you have glass tubes, a cup, a three-neck vial, thin-wire copper catalysts, a flat bottom flask and a pumped air supply, this might be worth a try.
But if you do not have it, it is not worth considering a system formed of metal for large quantities first if it is made with small amounts.
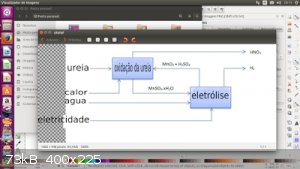
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Although extremely smelly, I used maggots with alot of sawdust on some road kill, maggots seem to produce alot of ammonia! then its a case of mixing
the sawdust with a little water and heating to recover the Ammonia.
I could have purchased the Ammonia, but when i tried it the point was OTC bush type chemistry. Worked ok and i was surprised just how much they
produce in 4-5 days, i found if i didnt use the sawdust to absorb the 'juice', the maggots died sometimes in the liquid. If you try it, do it outside
well away from humanity!! It stinks really bad, the Ammonia is strong enough to make your eyes water, i used a sealed bucket when i did it.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Would the process work with fat scraps (the maggot thing). I know that it gets really nasty smelling after about 4-5 days at room temp - or does it
need meat and skin?
What about rendered fat?
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
I think you'd enjoy the Ostwald post if oxidation of ammonia interests you.
https://www.sciencemadness.org/whisper/viewthread.php?tid=71...
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RogueRose  | Would the process work with fat scraps (the maggot thing). I know that it gets really nasty smelling after about 4-5 days at room temp - or does it
need meat and skin?
What about rendered fat? |
Should work I guess
Havnt you got a spare relative 
[Edited on 4-10-2017 by NEMO-Chemistry]
|
|
|
e.liska
Harmless

Posts: 42
Registered: 24-8-2017
Member Is Offline
Mood: Harmless
|
|
Quote: Originally posted by RogueRose  | Would the process work with fat scraps (the maggot thing). I know that it gets really nasty smelling after about 4-5 days at room temp - or does it
need meat and skin?
What about rendered fat? |
Fat does not contain much nitrogen, I would say you would need lot of proteins to produce ammonia.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by RogueRose  | Would the process work with fat scraps (the maggot thing). I know that it gets really nasty smelling after about 4-5 days at room temp - or does it
need meat and skin?
What about rendered fat? |
Neither of those have much in the way of protein/nitrogen in them, so you'd get very little ammonia. Back in the old days, they'd use the hooves,
horns, hair, and hides, since those are all mostly protein. Fat has plenty of more useful applications.
Incidentally, urea is actually really difficult to extract from urine. It's not nearly as easy as everyone seems to assume.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Melgar  | Quote: Originally posted by RogueRose  | Would the process work with fat scraps (the maggot thing). I know that it gets really nasty smelling after about 4-5 days at room temp - or does it
need meat and skin?
What about rendered fat? |
Neither of those have much in the way of protein/nitrogen in them, so you'd get very little ammonia. Back in the old days, they'd use the hooves,
horns, hair, and hides, since those are all mostly protein. Fat has plenty of more useful applications.
Incidentally, urea is actually really difficult to extract from urine. It's not nearly as easy as everyone seems to assume. |
I wonder if the "glue factory" could have also been a fertilizer factory as well? I remember hearing about entire horses and road kill from early
days being dissolved in large vats of something. Maybe they were making ammonia??
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by RogueRose  | | I wonder if the "glue factory" could have also been a fertilizer factory as well? I remember hearing about entire horses and road kill from early
days being dissolved in large vats of something. Maybe they were making ammonia?? |
Until the Haber-Bosch process made ammonia a commodity product, there weren't actually that many uses for it. After all, any form of nitrogen other
than N2 can be used as a fertilizer, so after animals would go through the rendering plants, whatever was left over would just be ground up and spread
on fields. There wasn't any need to separate it, since plants need phosphates, calcium, sulfur, potassium, etc. too.
Ammonia was used for cleaning and for dying cloth, but that could be obtained by pyrolysis of animal parts, as well as from urine.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
Attachment: INSTRUCTIONS for the manufacture saltpetre.docx (35kB)
This file has been downloaded 246 times
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I found this ages ago, thx to the new improved cataloging system on my pc  , it
has reappeared! So I thought as it was kind of on topic I would post it here for you, its all the info you ever need on maggots and ammonia
production, might just be me but i found it really interesting, however do keep in mind i am a bit odd. , it
has reappeared! So I thought as it was kind of on topic I would post it here for you, its all the info you ever need on maggots and ammonia
production, might just be me but i found it really interesting, however do keep in mind i am a bit odd.
Attachment: robinson1939.pdf (332kB)
This file has been downloaded 237 times
|
|
|