Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Deuterated Sulfuric acid as a Deuterium source for fusors.
Im planning on building a fusor and there is no way i will be able to get the money to buy a deuterium gas tube anytime soon so if i want to do fusion
i will need to make my own deuterium gas.
The cheapest source of deuterium is heavy water but you need relativly pure deuterium gas for fusors according to some posts on fusor.net. There are
some designs there for Deuterium Oxide electrolysis for generation of Deuterium gas but they are very complex and again i have no chance of doing it
on my own anytime soon.
So i have an idea for an alternate way of generating Deuterium gas. Acids reaction with metals is similar to that of electrolysis of water. If you had
some acid deuterated nonvolatile acid to react with a metal you could theoretically generate pure deuterium gas that way.
The acid should non-volatile, not produce volatile byproducts from the reaction with metals, have an easily prepared anhydride (so the deuterated acid
can be prepared from it) and waste as little deuterium as possible from side reactions and as byproducts.
Deuterated Sulfuric acid should fulfill this role nicely. At room temprature it has a vapor pressure of less than a pascal and sulfur trioxide can be
made OTC in various ways and reacted with Deuterium oxide to prepare the acid.
The metal should not be too reducing so it does not produce byproducts and the product from the reaction with the acid should be soluble in it.
Iron seems like a good choice for this because it is very easily handeled and not too reactive. Ferrous sulfate should react with the excess acid to
produce ferrous bisulfate which can be reasonably expected to be soluble.
I am not very good with vacuum systems and intend to learn as i start experimenting but here is an idea for a design. (Note that im trying to keep
machining to a minimum).
Will it work? Can you come up with some solvent for the acid and reaction products that will keep the Deuterium pure? What would be the best way to
make the deuterated Sulfuric acid?
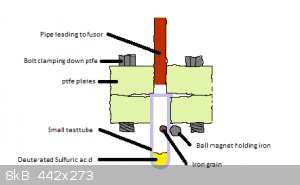
[Edited on 5-10-2017 by Σldritch]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
How would this be any simpler to set up than electrolysis? If you can build a fusor, surely you can manage to electrolyze heavy water.
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
It would replace a lot: http://www.fusor.net/board/viewtopic.php?f=24&t=3127, even the needle valve as the deuterium production can be regulated by moving the iron in
and out of the acid.
I probably can not build a traditional fusor, but, by simplifying it maybe i can build a fusor.
[Edited on 5-10-2017 by Σldritch]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Sulfuric acid+ iron creates hydrogen???
More like sulfur dioxide if you use concentrated acid.
If memory serves me right you can get a bit of sulfur dioxide with even somewhat dilute H2SO4.
Maybe zinc or tin would serve you better. Especially since you need a highly pure gas.
But really, electrolysis is going to be simpler to pull off.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
What about combining P2O5 with heavy water? That way you don't need oleum, and you don't have to worry about the metal reducing the sulfuric acid to
SO2 and metal/hydrogen sulfides. The only issue there is the low solubility of metal phosphates, although that might not be a bad thing as it allows
for easy removal of the byproducts.
Zinc and tin definitely will reduce concentrated sulfuric acid, often to H2S even.
edit: How important is it that water be kept out of the gas stream? Phosphoric acid is very hygroscopic, but phosphate salt buildup on the surface of
metals would prevent their further reaction. Is it more important that proteum (single-proton hydrogen) not be in the gas stream, or is it more
important that water be kept out of it? Or are you most concerned with the cost of heavy water?
One thing to consider is the purity of the metal you'd be using. Since iron is typically loaded with impurities, you should expect to get all sorts
of fun gases in your stream if you react that with an acid. Zinc is pretty bad too. Tin might be okay, but probably isn't reactive enough. Aluminum
has a lot of impurities, but you could try it anyway, since those impurities might not contaminate your gas stream. Magnesium seems like it might be
your best bet, for reacting with a nonvolatile acid like H3PO4.
[Edited on 10/11/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Texium
|
Thread Moved
27-11-2023 at 11:39 |