| Pages:
1
2
3
4
5 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
| Quote: | Sorry @Melgar, but I didn't understand the last part.
This reaction was extracted from a published book.
Do You believe that's impossible to occurs? The author is a fraud? yes or not? why? |
Hey guys, what's the consensus here on Jared Ledgard? I know he publishes a lot of books that are perhaps one step above Uncle Fester in apparent
legitimacy, but with the exact same subject matters. I never actually read any of the books myself, but I've heard they're mostly just compiled from
information he found online, with a lot of typos.
Oh, and that synthesis or whatever you have is obviously missing a step. There needs to be a Nef reaction or a dissolving metal reduction with
strongly acidic conditions (to reduce to the oxime then hydrolyze), if the intent is to perform a reductive amination.
It's not for every reaction, only certain ones. The Riemer-Tiemann reaction, for one. Like, it para-formylates guaiacol to give vanillin, and the
Ladenburg synthesis of piperine also does a Riemer-Tiemann formylation of catechol. I thought this was true of the Gattermann aldehyde synthesis as
well, but I think that's probably always para-directed. It could be that ortho/para formylations often give the ortho isomer as the major product
with phenol, and the para isomer as the major product with catechol.
The Duff reaction doesn't seem like it'd work. Ah well. And for those of you giving all sorts of not-very-OTC syntheses, here are the revised
criteria:
Reagents must be purchasable within the US
If something is sold by a retail store, it must be a chain store with a presence in at least half of the 50 states
If something is available online, it must be sold by at least three different vendors
Sellers on eBay and Amazon are considered "vendors" for this purpose. Note that the same seller (say, CCS) selling products on multiple
platforms only counts as one vendor.
Sorry for the list of rules, I just want this to be something that someone could theoretically reproduce without running into sourcing problems.
I've heard that ethylvanillin is easier to dealkylate than vanillin, but the references all seem to refer to some original Q document that I've been
unable to find. If it turns out that this is true, then this might be the way to go. Catechol isn't especially easy to source, and is more expensive
than ethylvanillin, so I may just discuss catechol for a minute or two and then proceed to do the synthesis with some vanillin-type aldehyde.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
His book King's Chemistry says you can do a reaction that closely resembles a Friedel-Crafts on catechol with allyl alcohol in acetone using potassium
carbonate as the catalyst instead of a Lewis acid:
| Quote: |
Page 96
Step 1: Preparation of 4-allyl catechol
Into a suitable reflux apparatus, place 13 grams (0.45 oz.) of catechol, followed by 14 grams (0.49 oz.) of allyl bromide, and then add in 22
milliliters (0.74 fluid oz.) of dry acetone. Then stir the entire mixture to form a uniform mixture. Immediately thereafter, gradually add in 17 grams
(0.59 oz.) of finely divided anhydrous potassium carbonate, and stir the mixture while adding this potassium carbonate. After the addition of the
potassium carbonate, reflux the entire reaction mixture at 60 Celsius for about 3 hours. Note: fit a calcium chloride drying tube to the top of the
reflux condenser to keep moisture out from the apparatus. After refluxing for about 3 hours, quickly remove the reflux condenser, and replace it with
a conventional cold water condenser, fitted with a receiver
Page 97
flask, and then distill-off the acetone until no more acetone passes over into the receiver flask. When this point is reached, stop the distillation
process, and allow the reaction mixture to cool to room temperature. Thereafter, pour the distilled reaction mixture left over, into a clean beaker,
and then add in 25 milliliters (0.84 fluid oz.) of cold water, followed by 100 milliliters (3.4 fluid oz.) of 10% sulfuric acid solution. Then stir
the entire acidic reaction mixture for about 10 minutes. Thereafter, extract the entire reaction mixture with one 50-milliliter portion (1.7 fluid
oz.) of diethyl ether. After the extraction process, wash the ether portion by adding to it, a sodium hydroxide solution prepared by adding and
dissolving 35 grams (1.2 oz.) of sodium hydroxide into 150 milliliters (5 fluid oz.) of water. Note: the addition of sodium hydroxide to water
generates much heat, so allow the mixture to cool to room temperature before using. Thereafter, remove the upper ether layer by using a seperatory
funnel, or by decantation, and then discard or recycle this upper ether layer (will contain diallyl ether). Now to the lower water layer, add in 100
milliliters (3.4 fluid oz.) of 10% sulfuric acid, and upon the acid addition, some oil should separate. After the addition of the sulfuric acid,
extract the entire acidic mixture (including any separated oil) with three 50-milliliter portions (three 1.7 fluid oz. portions) of methylene
chloride. Note: after each extraction, the methylene chloride will be the upper layer. After the extraction process, combine all methylene chloride
extracts, if not already done so, and then dry this combined methylene chloride mixture by adding to it, 15 grams (0.52 oz.) of anhydrous magnesium
sulfate—thereafter, stir the whole mixture for about 10 minutes, and then filter-off the magnesium sulfate. Thereafter, place the filtered methylene
chloride mixture into a distillation apparatus or rotary evaporator, and remove the methylene chloride. When no more methylene chloride is collected,
recover the left over remaining oil. Now, to this oil, place it into a reflux apparatus, and heat it to 180 Celsius. Note: during the heating process,
the oil will self heat raising the temperature to about 260 Celsius. When this temperature change results, stop the heating process, and then place
the oil (which will now be red in color) into a vacuum distillation apparatus (after it has cooled, or simply replace the reflux condenser with the
appropriate glass adapters and immediately begin the vacuum distillation process), as similar to the one used for the distillation of safrole as
listed above, but use only one condenser and receiver rather then two, and distill the oil at 158 celsius under a vacuum of 16 millimeters of mercury.
When no more oil is obtained at this temperature and vacuum, stop the distillation process, and then remove the left over remaining residue, and
discard it. To the collected fraction, re-vacuum distill it using the same apparatus (after it has been cooled, and cleaned), and re-vacuum distill
the oil at 158 Celsius, under a vacuum of 16 millimeters of mercury to obtain a refined 4-allyl catechol product. |
Does this actually work? It's questionable but interesting. Perhaps you might substitute vinyl bromide for allyl bromide if your target is dopamine
(and what happens if you substitute a Lewis acid for the potassium carbonate?). It's definitely sketchy, but he might have based this on solid
research that he neglected to cite.
He also devoted several pages to how to extract various natural products that might be used to make catecholamines. It's not clear whether he's
actually tried this himself.
Edit: The first time I read this I skipped reading most of the workup and missed the rearrangement part... the potassium carbonate is a catalyst for
forming the phenyl ether and is actually removed prior to the ring alkylation.
[Edited on 25-10-2017 by JJay]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It looks like allyl alcohol might be an active FC electrophile:
http://isivast.org.vn:8888/bitstream/123456789/12963/1/1501....
But unfortunately there are no intermolecular rxns using unsubstituted allyl alcohol on phenols. Looking around I did find an aurous-catalyzed
formation of chromans from allyl alcohols and phenols which may explain the lack of such rxns.
Melgar: You know how to make nitroethylene, correct? Nitromethane + formaldehyde + H2SO4. I'm not sure if my idea fell into the "not OTC" category.
Anyway I did some more searching and just as was the case with allyl alcohol there are loads of FC rxns with nitroethylene but they all use indoles or
pyrroles and no phenols. So nitroethylene F-C is probably out.
However, it turns out that organocopper reagents will react with nitroethylene and other nitroalkenes in the desired manner; see:
http://anonym.to/http://www.chem.ntnu.edu.tw/en/files/writin...
The reagents also react with allylic acetates. These zinc organocuprates can be prepared by transmetallation of organozinc reagents with copper (I)
salts, usually CuCN*2LiCl for solubility reasons because the only studies on the zinc-copper reagents use organozinc reagents generated in THF from
reactive organohalides and activated zinc.
However, arylzinc reagents can also be generated in acetonitrile, which was not known at the time that the original zinc-organocuprate studies were
being carried out. Generating arylzinc reagents in MeCN uses a cobalt catalyst:
http://anonym.to/http://pubs.acs.org/doi/abs/10.1021/ja02894...
Because copper (I) salts generally have much better solubility in acetonitrile (vs THF) it stands to reason that the transmetallation of
organozinc@acetonitrile to zinc organocuprate@acetonitrile should work just fine; unfortunately, it doesn't seem that anyone has tried to convert
these arylzinc reagents to cuprates. However, it seems like a relatively safe bet - as safe as any bets involving organometallic reagents can be
anyway. And ordinary copper (I) salts should be fine -- no need for cyanide.
The resulting organocuprate could then add to nitroethylene. It's not clear to me if the phenols need to be protected in order for this rxn to
proceed; organozinc and organocopper reagents are generally somewhat resistant to protons unlike their Li and Mg cousins. Also, phenols are very weak
acids in acetonitrile. I'm pretty sure the presence of catalytic amounts of cobalt will not interfere with the transmetallation, as long as the
arylzinc is generated before the copper salt is added.
The good news is that bromination of benzodioxole is high-yielding and can be achieved using NH4Br/H2O2 avoiding Br2:
http://anonym.to/https://erowid.org/archive/rhodium/chemistr...
And the most non-OTC thing here is CoBr2, which shouldn't be that hard to obtain in small amounts.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by JJay  | His book King's Chemistry says you can do a reaction that closely resembles a Friedel-Crafts on catechol with allyl alcohol in acetone using potassium
carbonate as the catalyst instead of a Lewis acid:
Does this actually work? It's questionable but interesting. |
Hey @JJay, I'm afraid it will not work. I suspect Jared Ledgard books are not so trustworthy as I have thought.
I found the same reaction you described from his book King's Chemistry Survival Guide at another book called Preparation of Organic Intermediates -
David Shirley - Wiley - 1952, between a phenol (cathecol in this case), allyl bromide, Potassium carbonate as a catalizer and acetone as a solvent.
That is quite the same reagents.
However, the product is an allyl phenyl ether and not an allyl phenol (cathecol in this case). The addition isn't like a friedel-crafts reaction, but
instead, a substitution of the phenolic group by an ether.
Take a look on this:
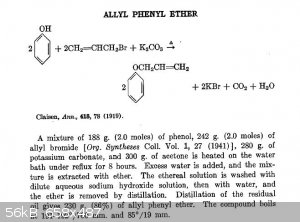
EDIT: Ok, I apologize, The allyl phenyl ether will rearrange (Claisen) to allyl phenol (cathecol) at high temperature. I see that now. Jared Ledgard
is not so stupid after all.
[Edited on 24-10-2017 by Chemi Pharma]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Pretty sure I've seen Shulgin pull this same reaction somewhere in PIHKAL for some compound where the ring substitution pattern couldn't be put
together by simpler means.
Looked it up: it's the 4-benzoloxy-3,5-dimethoxyamphetamine synthesis.
He does it to get the 5-methoxyeugenole intermediate.
Note: according to him this is a lousy drug that's not worth making anyway.
[Edited on 25-10-2017 by SWIM]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Chemi Pharma  |
The allyl phenyl ether will rearrange (Claisen) to allyl phenol (cathecol) at high temperature. I see that now. Jared Ledgard is not so stupid after
all.
|
I kind of skimmed the rearrangement procedure thinking it was just part of the workup... looks plausible, actually.
The $50,000 question is whether this will work with PVC monomer instead of allyl bromide.
[Edited on 25-10-2017 by JJay]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Doesn't the Claisen rearrangement have a cyclic intermediate?
If so, how would a 2-carbon phenol ether even reach?
Edit: for 'intermediate', read 'transition state'
I do know the difference on a good day.
[Edited on 25-10-2017 by SWIM]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Looks like you might as well just do a Friedel-Crafts.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Allylation of catechol is not applicable to dopamine whereas it is applicable to another compound we don't discuss, so I've refrained. However, be
assured that there is a route to 4-allylcatechol which involves a Claisen rearrangement. When there is a substituent ortho to the phenol, the Claisen
rearrangement can proceed to a 6,6-disubstituted cyclohexadienone, which rearranges to a para-substituted aromatic. I will not give conditions, but
with one ortho position blocked, the ortho:para ratio is about 1:1.
[Edited on 25-10-2017 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  |
It's not for every reaction, only certain ones. The Riemer-Tiemann reaction, for one. Like, it para-formylates guaiacol to give vanillin, and the
Ladenburg synthesis of piperine also does a Riemer-Tiemann formylation of catechol. |
neither of the two compounds is a catechol.In the RTR ,it is a known fact that if the ortho position is occupied(by methoxy,in guaiacol),the
formylation will occur para to OH.And in piperine synthesis,he formylates benzodioxole,which is again known to give para products
Quote: Originally posted by SWIM  | Doesn't the Claisen rearrangement have a cyclic intermediate?
If so, how would a 2-carbon phenol ether even reach? |
good catch ,all the examples I have seen use some form of allyl(or propargyl).Even if you could make the vinyl ether(which would be difficult), it
wouldn't rearrange.
My fries idea would give dopamine in 2 steps if glycine could be used
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Synthesis of 3,4-dihydroxybenzaldehyde(Protocatechuic aldehyde) from catechol
DE105798
US4165341
by Reimer–Tiemann reaction:
Reihlen; Illig; Wittig
Chemische Berichte, 1925 , vol. 58, p. 18
Reimer; Tiemann
Chemische Berichte, 1876 , vol. 9, p. 1269
Tiemann; Koppe
Chemische Berichte, 1881 , vol. 14, p. 2021
[Edited on 25-10-2017 by Waffles SS]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by CuReUS  | | neither of the two compounds is a catechol.In the RTR ,it is a known fact that if the ortho position is occupied(by methoxy,in guaiacol),the
formylation will occur para to OH.And in piperine synthesis,he formylates benzodioxole,which is again known to give para products
|
Well, I suppose it's possible to formylate in either order, but sources seem to indicate that the formylation came first. That would make sense,
since the Riemer-Tiemann reaction is the lower-yielding of the two, by quite a lot:
[img]http://slideplayer.com/slide/10398918/35/images/39/Piperine+was+synthesized+(Ladenburg,+1894)+by+the+reaction+of+the+piperic+acid+chloride+with+p
iperidine,+which+confirmed+the+structure+of+the+molecule.+The+synthesis+of+piperic+acid+was+achieved+starting+from+piperonal,+which+was+obtained+from+
catechol+using+Reimer-Tiemann+reaction+followed+by+the+condensation+with+diiodomethane+in+the+presence+of+a+base..jpg[/img]
Quote: Originally posted by SWIM  | good catch ,all the examples I have seen use some form of allyl(or propargyl).Even if you could make the vinyl ether(which would be difficult), it
wouldn't rearrange.
My fries idea would give dopamine in 2 steps if glycine could be used
|
Yes, glycine is certainly on the table. I'm sort of looking to use the most versatile reactions as possible for this synthesis, because it's supposed
to be educational though. I'm looking into everything though, but so much has been thrown on the table in this thread that it's taking a while.
@not_atara: Seems like a LOT of speculation. Could it work? Maybe. Would I expect it to work? Certainly not with me doing it.
It would be nice if there were easier ways to get chloroform, because I'd need to test the Riemer-Tiemann reaction at least 5 or 6 times before doing
it for real, and large excesses of chloroform always seem to be needed. Considering I get about 100 mL from a gallon of 8-10% sodium hypochlorite,
that's a lot of bleach to have to deal with.
By the way, what about ethylvanillin dealkylation? I'd like to try at least two routes just so I can verify that I did it correctly somehow.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Melgar  |
Quote: Originally posted by SWIM  | good catch ,all the examples I have seen use some form of allyl(or propargyl).Even if you could make the vinyl ether(which would be difficult), it
wouldn't rearrange.
My fries idea would give dopamine in 2 steps if glycine could be used
|
. |
In all fairness, the above quote is CuReUS.
I certainly don't deserve any credit for his Fries /glycine idea.
However I'm not too humble to point out that in the first part of that quote he's agreeing with me about something.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Glycine is an interesting idea, but it's not going to form a phenyl ester easily, and if you halogenate it or form an anhydride with it, it's going to
try to form peptides. Right?
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by Melgar  | | Yes, glycine is certainly on the table. I'm sort of looking to use the most versatile reactions as possible for this synthesis, because it's supposed
to be educational though. I'm looking into everything though, but so much has been thrown on the table in this thread that it's taking a while.
|
And what about protocatechualdehyde via aldimine with catechol, copper cyanide and HCl ? The formylation in this case is para oriented. I thought
about this after take a look on the preparation below:
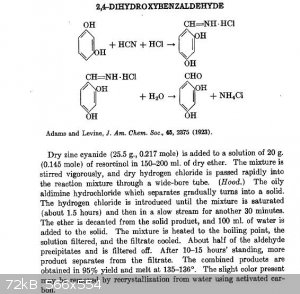
Also, the idea of @Waffles SS, brought through the patent US4165341 sounds brilliant, about sinthesize protocatechualdehyde from cathecol and
glioxilic acid with alumina as a catalizer with high yields (Patent in .pdf attached below)
Protocatechualdehyde is for me the best starting point, cause is only two OTC steps away from dopamine (Henry reaction with nitromethane and reduction
with sodium borohydride/nickel chloride in methanol).
[Edited on 26-10-2017 by Chemi Pharma]
Attachment: protocatechualdehyde from cathecol formylation with glyoxylic acid.pdf (689kB)
This file has been downloaded 369 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Fries rearrangement? Cool. But with glycine? I think you'd have to use N-protected glycine in order to prevent polymerization.
You could perhaps do the rearrangement with chloroacetic acid, then react the product with hexamine. But dimerization of alpha-ketoamones is an issue.
I'm not sure if you can make N-protected amines by alkylating cyanate anion in an alcoholic solvent, so the formed isocyanate converts to a carbamate
in situ. Succinimide or phthalimide would probably be the responsible choice here.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by Chemi Pharma  | | And what about protocatechualdehyde via aldimine with catechol, copper cyanide and HCl ? The formylation in this case is para oriented. I thought
about this after take a look on the preparation below: |
That's the Gatterman aldehyde synthesis that I mentioned earlier. It's zinc cyanide though, not copper cyanide. Incidentally, this is similar to a
FC acylation, but uses HCN as the source of the formyl group. To keep from having to use HCN gas, it's instead introduced as Zn(CN)2, then reacted
with HCl gas. This produces both the Lewis acid and the HCN in situ.
| Quote: | | Also, the idea of @Waffles SS, brought through the patent US4165341 sounds brilliant, about sinthesize protocatechualdehyde from cathecol and
glioxilic acid with alumina as a catalizer with high yields (Patent in .pdf attached below) |
Yep, this is similar to how vanillin is made. The trouble is getting glyoxylic acid. Supposedly that can be prepared from magnesium and oxalic acid,
but the reaction is very low-yielding if it yields anything. Another preparation mentions oxidizing ethanol with nitric acid, which is much more
likely to turn into a nitric-acid geyser than it is to give any glyoxylic acid. Electroreduction of oxalic acid might have to be the way to go here,
and supposedly this was how it was made industrially until recently.
| Quote: | | Protocatechualdehyde is for me the best starting point, cause is only two OTC steps away from dopamine (Henry reaction with nitromethane and reduction
with sodium borohydride/nickel chloride in methanol). |
I'm unsure of how acidic phenols affect the Henry reaction, although I would expect that you would just need more catalyst, or longer reaction times.
That wouldn't be my first choice of reduction methods though. First, because NaBH4 isn't easy to get, and second, because the nickel boride reaction
isn't as nice as you probably think it'd be. However, reducing with zinc and HCl can give the amine for nitrostyrenes, provided temperatures are kept
below 0C, which is probably the reduction method I'd use.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@Melgar, I have cited copper cyanide cause is more OTC. At least here, in south america, I can buy it withouth any restrictions applicable to alkaly
cyanides for just US$30,00 for 500 grs. I don't know about US restrictions, by the way. To this reaction it can be zinc or copper or even sylver
cyanide. I think every transition metal cyanide will work.
Glyoxylic acid 50% solution w/w is so cheap here that I'm ashamed saying the price: US$18,00 a liter. Glyoxylic acid is sold by perfumary, soap and
flagrancies stores, cause it's used as an exfoliating agent to the skin and to make facial masks. I guess you can find the 50% solucion w/w in US easy
if you find at this places, or at the e-bay, and not at a chemycal store. So, just buy it instead synthesize.
Henry reaction between nitroethane with protocatechualdehyde is extensed covered by many and many experiments you can read at Rhodium pages and at The
Hive. Many guys have registered their suscessful experiments on doing that, with no damage to the phenolic radicals. Care must been taken with
benzodiaxole ring, cause it's acidity sensibility, but it's not the case here. I think Nitromethane will behave the same way.
About reduction, borohydride here is easy to get (I have almost 500 grs right now), but I agree with you it's not so OTC if you want to make an
educacional video. I never trust in Zn + HCl to reduce nitroalkenes to give high yields. I suggest you, then, Tin + HCl or SnCl2 + HCl, that's a
classic and proved way to reduce them with razonable yield.
[Edited on 26-10-2017 by Chemi Pharma]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
First of all, zinc isn't a transition metal. Just figured I'd point that out. Also, the only transition metal that I regularly see used as a FC
catalyst is iron. Copper, I'd expect to be too prone to oxidizing things, and I'm not sure about its solubility in its anhydrous form. Now that I
think about it, I wonder if potassium ferricyanide or ferrocyanide could do this like zinc? Precipitate potassium as KCl, free cyanide as HCN and
iron would be FeCl3. That may be too much cyanide though, even if it did work.
Quote: Originally posted by Chemi Pharma  | | Glyoxylic acid 50% solution w/w is so cheap here that I'm ashamed saying the price: US$18,00 a liter. Glyoxylic acid is sold by perfumary, soap and
flagrancies stores, cause it's used as an exfoliating agent to the skin and to make facial masks. I guess you can find the 50% solucion w/w in US easy
if you find at this places, or at the e-bay, and not at a chemycal store. So, just buy it instead synthesize. |
Are you sure you aren't thinking of GLYCOLIC acid? Because that's exactly what you seem to be describing.
| Quote: | | About reduction, borohydride here is easy to get (I have almost 500 grs right now), but I agree with you it's not so OTC if you want to make an
educacional video. I never trust in Zn + HCl to reduce nitroalkenes to give high yields. I suggest you, then, Tin + HCl or SnCl2 + HCl, that's a
classic and proved way to reduce them with razonable yield. |
Zinc will only give good yields if temperature is kept cold, below 0C. Also, it has to be a nitrostyrene, not a nitropropene.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@Melgar,
I'm pretty sure it's Glyoxylic acid and not Glycolic acid.
I apologize, Glyoxylic acid is used for hair straightening and Glycolic acid is used for skin exfoliation. I misundertood the usage of the both. You
are right!
I didn't have it yet at my storage lab and I just have bought hours ago at the Net a 500 ml bottle for U$9,28 + mail expense, to do experiments with
cathecol like said at the Patent.
Here's the link I bought it in Brazil:
https://lista.mercadolivre.com.br/acido-glioxilico#D[A:acido-glioxilico]

AliBaba has the same 50% solution w/w to sell worldwide below U$10,00 per Kg:
https://www.alibaba.com/trade/search?fsb=y&IndexArea=pro...
[Edited on 26-10-2017 by Chemi Pharma]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | Fries rearrangement? Cool. But with glycine? I think you'd have to use N-protected glycine in order to prevent polymerization.
You could perhaps do the rearrangement with chloroacetic acid, then react the product with hexamine. But dimerization of alpha-ketoamones is an issue.
I'm not sure if you can make N-protected amines by alkylating cyanate anion in an alcoholic solvent, so the formed isocyanate converts to a carbamate
in situ. Succinimide or phthalimide would probably be the responsible choice here. |
Sigma has an absolutely massive selection of amino protecting reagents. I wonder if you could make one with cyanuric acid. Although succinimide can
certainly be found....
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Apparently glyoxylic acid hair straightening is a real thing, see e.g.:
http://onlinelibrary.wiley.com/doi/10.1111/ics.12148/abstrac...
JJay: I think 5,5-dimethylhydantoin is a better choice than cyanuric acid if you want things to be OTC. Only one of the protons on hydantoin is
removed at normal pH ranges, which prevents having to deal with polysubstituted things like 1,3,5-triazane-2,4,6-trione-1,3,5-triacetic acid".
5,5-dimethylhydantoin is the reduction product of BCDMH the common source of pool bromine.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I do think it would be easier to find suitable solvents for hydantoins.
It seems to me as though the rules say that the least OTC part should be the catechol. I cleaned out my fume hood yesterday and am tempted to play
with some aspirin to see if I can't get some catechol out of it... but I have a lot of other experiments I want to do, and it would be a lot less time
consuming to just buy it.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I worked with glyoxylic acid for more than 3 years.i tried many method for synthesis it.(Periodate method , Electroreduction method, Ozone method ,
Glyoxal oxidation method, ...).
I believe Easier method is Glyoxal oxidation by Nitric acid(industrial method).
Dispose of Nitrogen oxide is problem of this method that i solved it by different trap

Reaxys search attached
Attachment: glyoxylic acid.pdf (1.7MB)
This file has been downloaded 718 times
[Edited on 27-10-2017 by Waffles SS]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JJay  | | This is the first time I have heard of the Fries rearrangement... interesting. Why doesn't the chloro acetylchloride react with pyridine ?
|
because it can react more easily with the exposed,protruding OH rather than try to react with the flat,boxed in N.Also the lone pair of N is in
resonance making it less juicy
I found some methods to make the ester
http://orgsyn.org/demo.aspx?prep=cv2p0310 ( see the 1st and 2nd refs given)
http://www.prepchem.com/synthesis-of-glycine-ethyl-ester-hyd...
[Edited on 27-10-2017 by CuReUS]
|
|
|
| Pages:
1
2
3
4
5 |
|