| Pages:
1
2 |
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
Diclhloromethane synthesis
Please someone help with Dichloromethane Synthesis by Chloroform
I can't buy only with firm ,and is strictly controled.
In Eu-country the DCM was banned and in paint strippers was replaced
I've seach all over the internet
Thank you
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
As far as I'm aware, there is no simple process that will allow conversion of chloroform to DCM.
If you meant that you want to prepare DCM via a haloform reaction (as we would for chloroform), then this is not possible either. After the addition
of two chlorines to the methyl ketone, the remaining alpha-proton is so acidic that the reaction cannot be stopped at this stage (this intermediate
reacts much faster than any other electrophile in the reaction mixture). Besides, even if this wasn't a problem, [CHCl2]- isn't
a sufficiently good leaving group to allow DCM to be formed.
[Edited on 5-11-2017 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
only way is gas phase reaction in furnace tube under moderate pressure, can't recall if catylest was required or not but sure others will fill in
blanks
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Simple DCM synthesis is not a viable option, but perhaps chloroform could be used as a replacement....if it is to be used as a generic extraction
agent for separating organics from water based systems then quite often it can be used instead of DCM. It will depend of cource on the particular
purpose and combination of reagents in question.
Apart from that there are other solvents and solvent mixtures available OTC which can be used for generic extraction puposes: alkene mixtures, heavier
ester mixtures, xylene etc.... Perhaps some of these could be a suitable replcament?
Exact science is a figment of imagination.......
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It ought to be possible to do some sort of exchange reaction between methane and chloroform at high temp in the gas phase.
Not sure it's worth it.
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
DCM synthesis
I have read somewhere about a reduction of Chloroform :
Chloroform + Zn + HCl in Ethanol ..but I can't find anything about
I've spend all the night to search online and in chemical books
I know about synthesis by Methane,but is very complicated,and is needed a real industy investition
Thank you to all
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
DCM can also be prepared by reducing chloroform with hydrochloric acid and zinc metal in ethanol.
CHCl3 + Zn + 2 HCl → CH2Cl2 + ZnCl2 + HCl
http://www.sciencemadness.org/smwiki/index.php/Dichlorometha...
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
http://www.nature.com/nature/journal/v319/n6051/abs/319308a0...
What about that??
It is achievable?
Thank you
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
https://books.google.com/books?id=pdcBAAAAYAAJ&pg=PA522
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
Thank you,but what is in that book ?
I don't see the synthesis
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
I've found a gas tank with refrigerated agent DCM
Can I condense the gas to get DCM in liquid form ?
What about the freon disoved in DCM?
I think the freon is tha carrier gase
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Quote: Originally posted by dan.vlad  | I've found a gas tank with refrigerated agent DCM
Can I condense the gas to get DCM in liquid form ?
What about the freon disoved in DCM?
I think the freon is tha carrier gase
|
Are you sure about that? I've never heard of DCM being used as a refrigerant. Have you checked how much DCM is in the tank?
Batoussai.
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
DCM is used as refrigerant from long time
Is no more alowed to use,but I've found ..
My answer is ,how I can condese to get DCM in liquid form?
In DCM is also F ..
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/Dichlorodifluoromethane
The refrigenrant is known under the name R12
I think can I condese the gas from the cylinder wiht a good condenser coil with negative temperature
How can I get out the Hydrogen Fluoride?
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
R 30 refrigerant is DichloroMethane
https://www.researchgate.net/publication/283410683_R30_An_ov...
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
https://www.phenomenex.com/Compound?id=R30+(Refrigerant)
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
I just distill DCM out of paint stripper, then wash out any methanol. Dry with MgSO4, then redistil for purity. I even redistil the MeOH, to get a
technical grade stuff good for rinsing glassware.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
If you ABSOLUTELY need CH2Cl2 and CHCl3 won't do, then I imagine it's for a reaction and not a solvent, in which case you might want to look into
anhydrous cleavage of various forms of polymerized formaldehyde using hydrogen halides. You might find that CH2Br2 suits your need even better than
the chloride version?
Ugh. R12 is Freon. You know, that ozone-destroying shit that actually got the world to cooperate enough to enact the Montreal Protocol.
Difluorodichloromethane. There's no hydrogen fluoride in there, and if there was, you'd probably kill yourself getting it out. HF
is usually obtained by reacting CaF2 with H2SO4, but I highly recommend against attempting that any time soon.
I'm actually wondering now, if something like the Riemer-Tiemann reaction could be done in the presence of ammonium formate or something else that
easily loses hydrogen without a subsequent reaction with the rest of the reactants? Maybe Pd/C under hydrogen pressure, even? It'd be kind of
pointless, but sometimes these thought experiments can be fun.
[Edited on 11/7/17 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
DCM is one of those things that you either Buy or Don't.
Some chemicals you can make in a reasonable time/with reasonable effort, some you can't.
DCM is one of the 'not worth the trouble' types.
If it's for a solvent, re-think your process and choose another - there are many, some of which Can be made in an amateur environment.
Edit:
Is there Any procedure that you think you Need DCM for, or do you just want to have a bottle on the shelf labelled 'DCM' ? (better in the fridge
really).
[Edited on 7-11-2017 by aga]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
isnt ethyl acetate considered the more 'green' solvent for many extractions like caffeine?
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
That's interesting.
You'll end up with a DCM and Trichloromethane mixture.
DCM = bp = 39.6 °C
Trichloromethane = bp = 56-57 °C
You separate them by distillation.
Also, there was someone who made this stuff with methane and Cl2 + a powerful UV source. As a bonus, you get CCl4.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
http://en.wikipedia.org/wiki/Dichloroacetic_acid
According to Wikipedia, chloral reacts with cyanide in the presence of base (calcium carbonate) to produce dichloroacetic acid. Dichloroacetic acid
can then probably be decarboxylated to give DCM somehow. I assume this takes place via dehydrohalogenation of the cyanohydrin:
Cyanohydrin formation: Cl3CC(=O)H + HC#N >> Cl3CCH(OH)C#N
Dehydrohalogenation: Cl3CCH(OH)C#N + B- >> BH + Cl- + Cl2C=C(OH)C#N
Keto-enol tautomerism: Cl2C=C(OH) >> Cl2HCC(=O)C#N
Acyl cyanide hydrolyses: Cl2HCC(=O)C#N + H2O >> Cl2HCCOOH + HCN
Cyanide is probably catalytic if I'm right about the mechanism. It's possible that thiamine could act as an alternative catalyst for this reaction
because it forms an adduct with aldehydes which is similar to the cyanohydrin. If not, small amounts of cyanide may be prepared from formaldoximes as
described here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=23...
EDIT: the reaction is on Orgsyn, I was right, it is catalytic in cyanide:
http://www.orgsyn.org/demo.aspx?prep=CV2P0181
Another compound which has been used as a "nontoxic cyanide" is 1,3-dimethyl-1,2,4-triazolium. I'm not sure how best to synthesize this, however. I
think you can make N-phenyltriazole by the rxn of phenylhydrazine with formamide, which is a start....
[Edited on 9-11-2017 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
As it turns out chemplayer actually investigated the preparation trichloromethane from trichloroacetic acid and just decarboxylated the sodium acetate
salt thermally with a yield of 58%.
https://www.youtube.com/watch?v=K0Z_jZd7OeA&spfreload=5
They also investigated the dehalogenation of trichloroacetic acid with zinc.
Curiously they didn't activate the zinc metal before hand.
https://www.youtube.com/watch?v=RDhhJCk2ZbI
If you do intend to try the dehalogenation of chloroform then i would personally suggest activating it first by washing with dillute HCl then water
then ether then drying the shit out of it in a desiccator.
activated zinc dust doesn't keep well and will oxidize within a 24 hour span so use it quick or store under an inert atmosphere.
If the dehalogenation fails with activated zinc then i would possibly suspect its a flop.
@dan.vlad I assume you have the necessary equipment to carry out a fractional distillation?
[Edited on 9-11-2017 by Assured Fish]
|
|
|
dan.vlad
Harmless

Posts: 31
Registered: 5-11-2017
Member Is Offline
Mood: No Mood
|
|
Anyone knows who wrote the page? http://www.sciencemadness.org/smwiki/index.php/Dichlorometha...
At the bottom of the page is a reference,about the DCM synthesis " Organic Chemistry, RL Madan, Tata McGraw-Hill Education, pag. 379"
I think if it's written in the book the synthesis exists
I searched for the book but,I found two of the same author with the same name,but different covers
I've mailed the author " rattanlal.madan@gmail.com " but no response..
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
I got that reaction from the book when its preview was available through Google books, but later it was removed. I think I might have the book
somewhere on my computer, I'll need to look for it. Unfortunately, the reaction in the book didn't have a source, and no mention of any other reaction
conditions.
So back then when I had access to Reaxys I did a small search and found a few reactions which involved the reduction of chloroform with Zn.
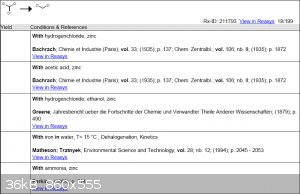
Unfortunately I can't access the first 3 articles online, I'm not even sure if some are in digital format.
However, I was able to access the article for the third reaction, and got this interesting bit:
| Quote: |
Carbon tetrachloride and chloroform undergo rapid reductive dehalogenation in the presence of fine-grained iron metal. With each successive
dehalogenation, the reaction proceeds more slowly, and methylene chloride was not significantly degraded over the time scales of our experiments.
Relative product distributions do vary with conditions however, so it is possible that circumstances may exist where significant degradation of
methylene chloride will occur. |
So it would appear that both chloroform and carbon tetrachloride can be rapidly dechlorinated by iron powder in water to dichloromethane, which
resists further dechlorination, with little degradation even after a month.
Now I'm curious if this method can be scaled up.
|
|
|
| Pages:
1
2 |