| Pages:
1
2 |
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
A Pump capable of Pumping Exotic Fluids
Some work I am doing on a specific project requires that a condenser be charged with a circulating cryogenic coolant. Being only an amateur, and not
with an institution just yet, I plan on making this coolant using dry ice and some volatile solvent: dry acetone, ethanol, or isopropyl alcohol. Dry
ice and acetone slurrys according to Wikipedia have a temperature of about 78C.
As a side not I once tried to preserve germinating soy seeds using varying solutions of ethanol and water inside a freezer @-80C but none survived.
What pump would be capable of moving acetone @-79C into a condenser and out, back into the basin? Does anyone have experience with this sort of work?
I am aware of the ricks involved in using any flammable liquid. The area will be cleared of ignition sources and ventilated.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
My instinct would be to go for a peristaltic pump. But at those temperatures you might have fun finding suitably flexible hosing.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by VSEPR_VOID  | Some work I am doing on a specific project requires that a condenser be charged with a circulating cryogenic coolant. Being only an amateur, and not
with an institution just yet, I plan on making this coolant using dry ice and some volatile solvent: dry acetone, ethanol, or isopropyl alcohol. Dry
ice and acetone slurrys according to Wikipedia have a temperature of about 78C.
As a side not I once tried to preserve germinating soy seeds using varying solutions of ethanol and water inside a freezer @-80C but none survived.
What pump would be capable of moving acetone @-79C into a condenser and out, back into the basin? Does anyone have experience with this sort of work?
I am aware of the ricks involved in using any flammable liquid. The area will be cleared of ignition sources and ventilated. |
I would use a peristaltic pump and decent silicon tubing, but others more qualified might have a better idea.
Thats pretty cold for a condenser, cant you lower the pressure and use a cold trap?
Some the smart people will have some ideas for you
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Quote: Originally posted by NEMO-Chemistry  |
I would use a peristaltic pump and decent silicon tubing, but others more qualified might have a better idea.
Thats pretty cold for a condenser, cant you lower the pressure and use a cold trap?
Some the smart people will have some ideas for you |
Do you have another commendation for a coolant? The condenser needs to be able to condense butane which liquefies/boils at about 0C.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
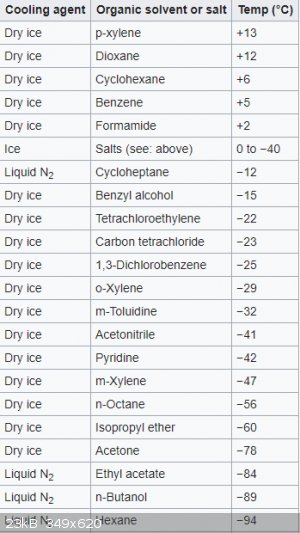
https://en.wikipedia.org/wiki/List_of_cooling_baths
Looks like ice/acetone will work for you.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
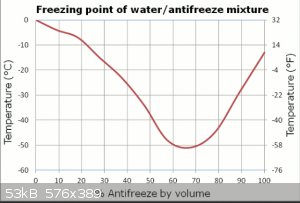
I would go with a peristaltic pump also but would try ethylene glycol (mp = -12.9°C) as coolant.
Mixtures of ethylene glycol and water could get you much cooler, IIRC.
Any centrifugal pump capable of handling ethylene glycol for auto engine cooling should work, say one made of steel for example.
[Edited on 10-11-2017 by Magpie]
[Edited on 10-11-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
A peristaltic pump with Chemflex (Gore) tubing will survive that, not much else will flex at -78C. It costs an arm and a leg, but will survive
anything I have every tried. Silicone might survive, but not for long, I suspect.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
A few months ago I bought some peristaltic pumps for use with my distillation rig,
six ex-dairy pumps for GBP24 total 
I replaced the tubing with the correct size of silicone tubing as it is cheap enough to replace frequently if required.
HOWEVER, acetone will quickly destroy silicone tubing.
Could you use ice/salt/water ?
It should be just cold enough if you go slowly I think.
I find that even ethanol at RT slowly softens silicone, maybe ok at iced temperatures ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Standard Viton A is good to -17°C and is good for ethylene glycol.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
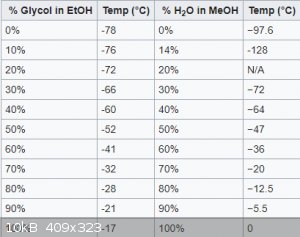
I found some information regarding dry ice and various coolants. Could a aquarium pump handle temperatures of -10C to -20C?
[Edited on 10-11-2017 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Yes, I expect the temperature would not be a problem. However, depending on the pump composition, whatever solvent you're pumping might degrade the
components.
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Ethylene glycerol and water might be the solution then
[Edited on 10-11-2017 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
A marine aquarium pump (various sizes) would hold out no problem with salt water. I dont think a quality pump like say a Ehiem marine pump would
suffer with that.
the parts are made to handle salt and last a long time, the datasheet should give ideal working temps. Add in a safety margin and i bet -10-15 is no
problem
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Pumping butane however for extracting exotic chemicals from plants, often goes bang 
Recycling or using a soxhlet would be novel, but if your working with plant material, they already make a device to collect the CO2 or butane after
extraction, its a two pot system.
Dosnt cost that much from memory
[Edited on 10-11-2017 by NEMO-Chemistry]
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Pumping around dry ice cooling baths is often very difficult in practice. The solutions are saturated with carbon dioxide, as soon as they hit a low
pressure region of the pump or start to warm up they start to off-gas which will cause some pumps to become air-bound or just result in hardly
anything moving other than the gas.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
http://www.scisolinc.com/pre-owned-equipment
There is a smaller cheaper version around if you look on alibaba. If your extracting what I think you are, Butane is taking a risk. For what it costs
V profit... CO2 is much safer
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Quote: Originally posted by NEMO-Chemistry  | Pumping butane however for extracting exotic chemicals from plants, often goes bang 
Recycling or using a soxhlet would be novel, but if your working with plant material, they already make a device to collect the CO2 or butane after
extraction, its a two pot system.
Dosnt cost that much from memory
[Edited on 10-11-2017 by NEMO-Chemistry] |
I am not pumping butane. I wish to basically have the butane reflux. Its the condenser that need to have very cold liquid circulated through it.
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by VSEPR_VOID  | Quote: Originally posted by NEMO-Chemistry  | Pumping butane however for extracting exotic chemicals from plants, often goes bang 
Recycling or using a soxhlet would be novel, but if your working with plant material, they already make a device to collect the CO2 or butane after
extraction, its a two pot system.
Dosnt cost that much from memory
[Edited on 10-11-2017 by NEMO-Chemistry] |
I am not pumping butane. I wish to basically have the butane reflux. Its the condenser that need to have very cold liquid circulated through it.
|
I got that in the end, hence the soxhlet reference. I take it your basically doing SCE and what to reuse the Butane?
As i linked to there are devices to do just this. Smaller than i linked too and on alibaba.
It extracts in one side then goes into the other where through pressure or whatever gets re condensed. But most use CO2 as its safer.
I cant see how your going to reflux it though. And considering how it works, refluxing is redundant isnt it?
[Edited on 10-11-2017 by NEMO-Chemistry]
[Edited on 10-11-2017 by NEMO-Chemistry]
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Nerd rage recommends antifreeze and aquarium equipment
[Edited on 11-11-2017 by VSEPR_VOID]

Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Why not just use a cold trap/finger type of condenser ?
this would not need a pump
e.g. https://www.ebay.co.uk/itm/Condenser-BUCHI-Cold-Finger-Glass... pwAAOSwZ4dZJUbb pwAAOSwZ4dZJUbb
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I still dont understand how this will work???
Lets assume he is extracting oils from plant matter with Butane. once the liquid butane has passed through the material, all the oil has been
extracted. So how are you going to then recover the Butane with out a fairly complicated system? A cold finger or even refluxing serves no point.
Once the liquid has gone through the material its job done, i am pretty sure he then wants to recover the butane. However I except he did mention
reflux, but when I tried Critical extraction with CO2, it takes one pass. The liquid is gas by default as it passes through the material, i thought
that was actually how critical extraction works.
TBH i think the reason for lack of clarity is down to the material being a certain herb. However personally i dont think that matters, but i think it
matters if he is actually trying to do a critical extraction. Purely because recovery i would think very hard unless a stainless steel two pot system
is used.
But I am probably wrong, my own experience was with mint and tomato plants. Extraction was good but recovery i would think is pretty hard. Also if it
is plant extraction then Butane is a risk. You dont need much gas to process alot of material, i would personally use CO2 and not try to recover, its
not worth it.
@Sulaiman
Yes I think the coldfinger you link too would work, but if the idea is simply reflux plant material, then its pointless? Refluxing wont recover more
material. I also think that cold finger would need dry ice to work well.
BTW i am aware i sound arsey, this isnt intended at anyone, i got toothache  . .
[Edited on 11-11-2017 by NEMO-Chemistry]
[Edited on 11-11-2017 by NEMO-Chemistry]
[Edited on 11-11-2017 by NEMO-Chemistry]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Hash oil manufacture is legal in my jurisdiction. Really, not a problem!
Manufacture in a residential area, is a serious felony. To prison you will be a-going.
Too many explosions, fires, and deaths.
http://www.oregonlive.com/portland/index.ssf/2017/07/im_so_s...
Using butane in such a manner, is a bad idea. Find a better solvent.
Isopropyl Alcohol, is said to work acceptably well.
As for pumping -78C solvent, through a condenser...... Again, a bad idea.
Find a simpler solution.
https://www.google.com/search?q=scully+lsd+diagram&tbm=i...
This is Tim Scully's diagram for a flash evaporator. A solution of product in chloroform, is injected into a vacuum chamber. Product is left in
chamber, and Chloroform is instantly vaporized (hopefully) and drawn towards distant condenser, still under vacuum of course. The evaporation chamber
is heated by a circulating current of COLD tapwater around the chamber (evaporation does suck up heat ) Chloroform vapor passes through the apparatus
and is condensed in a large flask, submerged in dry ice and acetone.
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | Hash oil manufacture is legal in my jurisdiction. Really, not a problem!
Manufacture in a residential area, is a serious felony. To prison you will be a-going.
Too many explosions, fires, and deaths.
http://www.oregonlive.com/portland/index.ssf/2017/07/im_so_s...
Using butane in such a manner, is a bad idea. Find a better solvent.
Isopropyl Alcohol, is said to work acceptably well.
As for pumping -78C solvent, through a condenser...... Again, a bad idea.
Find a simpler solution.
https://www.google.com/search?q=scully+lsd+diagram&tbm=i...
This is Tim Scully's diagram for a flash evaporator. A solution of product in chloroform, is injected into a vacuum chamber. Product is left in
chamber, and Chloroform is instantly vaporized (hopefully) and drawn towards distant condenser, still under vacuum of course. The evaporation chamber
is heated by a circulating current of COLD tapwater around the chamber (evaporation does suck up heat ) Chloroform vapor passes through the apparatus
and is condensed in a large flask, submerged in dry ice and acetone.
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed]
[Edited on 11-11-2017 by zed] |
Thx
That would work well for small volumes of plant oils I work with. I am after tomato leaf extract, basil and that kind of stuff. save with all the
other gunk you get with straight solvent or water extractions.
But should work really well with pure Rose oils, those are high value and normally Critical extraction with CO2. I might try next summer with this
method.
Hash oil in UK is illegal and a class 1 drug! hash plants can get you serious prison time, which is a shame as its a really fascinating plant.
Chemically I find it fascinating also, but no chance working with it.
Is not worth the risk for me to grow or use hash, regardless if its not for recreation.
[Edited on 12-11-2017 by NEMO-Chemistry]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, working with food products, it is nice to have no solvent residue in the final product. Scully and friends were working with a temperature
sensitive, UV sensitive product, in a fairly toxic solvent. They cleaned it up, famously well.
The nice thing about flash evaporation or freeze drying, is that you can probably strip off essentially all of the solvent, quickly at low
temperatures. Spraying a mist, into a vacuum, presents a tremendous surface area, which is rapidly and thoroughly evaporated of solvent.
Alternately, folks often utilize a rotary evaporator for such extract concentrations. But, more heating is generally required, and the apparatus
itself may be pretty expensive. Here in the U.S., Gourmet chefs have taken up this practice.
If you can produce a reasonable vacuum, you can probably improvise a flash evaporator of sorts, from odds and ends of glassware.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Alternately, for some extractions. Liquifying via Vitamix or other high speed blender, straining, and then freezing out the desired product is a
useful approach.
Did it recently with egg-plant. Upon freezing the liquid, concentrated brown goo is extruded from the iceblock. Homemade Curaderm.
https://joyofblending.com/vitamix-speed-measured/
[Edited on 14-11-2017 by zed]
|
|
|
| Pages:
1
2 |