adianadiadi1
Harmless

Posts: 3
Registered: 20-11-2017
Member Is Offline
Mood: No Mood
|
|
Stereochemistry of product in Grignard-Gilman addition reaction
I would like to know whether the stereochemistry given in the following reaction is correct or not?
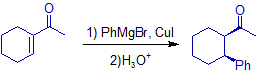
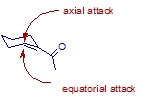
Which attack is more likely? axial or equatorial?
[Edited on 20-11-2017 by adianadiadi1]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Cyclohexene derivatives are not in a true chair conformation, hence there is no such thing as an axial/equatorial attack. Cyclohexene derivatives
usually have two energetically favorable half-chair conformations the 3H4 and the 4H3. Since the drawn
cyclohexene derivative is unsubstituted both states will be populated to an equal extent. Attack on these half-chairs can only occur from the bottom
because the top-side would result in a twist boat conformation which is energetically unfavorable.
Based on this I would conclude there is no preference for one enantiomer, as both states are populated to an equal extent.
When compounds are drawn as a single diastereomer the enantiomer of said diastereomer is taken to be formed to an equal extent. (Most correct would be
using the ± sign along with the drawing of the single diastereomer but this is often omitted). Now then onto the question which of the diastereomer
is formed?
This is determined by on which face the enolate gets protonated in the acidic work-up. Since a proton is small I suspect there is no difference
between the axial and the equatorial attack and result in an equal mixture of both enantiomers at the alpha position, i.e. a racemic mixture.
Protonation, depending on conditions can be reversible. If this is so the reaction will be under thermodynamic control and I suspect the
equatorial-equatorial product will be the dominant one. This is not the product you've drawn.
A more simple way of looking at this is: Is chirality induced by the substate? No. Is it induced by the use of chiral reagents? No. Does magic happen?
No. Thus the result will be a racemic mixture.
[Edited on 20-11-2017 by Sigmatropic]
[Edited on 20-11-2017 by Sigmatropic]
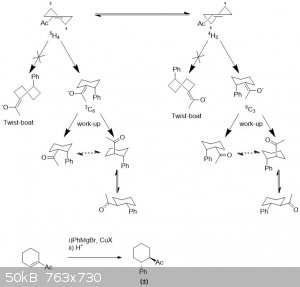
[Edited on 20-11-2017 by Sigmatropic]
|
|
|
gdflp
|
Thread Moved
20-11-2017 at 10:44 |
adianadiadi1
Harmless

Posts: 3
Registered: 20-11-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sigmatropic  | Cyclohexene derivatives are not in a true chair conformation, hence there is no such thing as an axial/equatorial attack. Cyclohexene derivatives
usually have two energetically favorable half-chair conformations the 3H4 and the 4H3. Since the drawn
cyclohexene derivative is unsubstituted both states will be populated to an equal extent. Attack on these half-chairs can only occur from the bottom
because the top-side would result in a twist boat conformation which is energetically unfavorable.
|
Thank you. May I know why the twist boat is formed when the attack is from the top? What if the carbon is pushed down when Ph approaches it? We get
chair too..........?
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
If you push down that carbon while the phenyl attacks on top you get some seriously distorted geometry at that carbon (almost like an umbrella instead
of the tetrahedron). But yes this twist boat can become a chair, but this does not affect the transition state which still leads to a twist-boat.
I'm starting to think the second part of my answer is wrong. I'll make a new one once I make up my mind.
|
|
|