BezeneBoy
Harmless

Posts: 3
Registered: 27-11-2017
Member Is Offline
Mood: No Mood
|
|
TetraChloroMethane Using Carbon and Chlorine ?
I hope this is the right thread;
I know that fluorine gas will spontaneously ignite carbon to form oxidation products that are Fluorocarbons,
Hence under the right conditions one could expect chlorine to oxidise carbon to Chlorocarbons, one of which, would be tetrachloromethane, a useful
solvent. I can't seem to find any resources on this as the production of TCM is performed using methane gas and the free radical mechanism.
I'd imagine it would require a very complicated setup, but I don't see any evidence pointing against the fact that chlorine will oxidise the carbon
much like oxygen does in regular combustion.
Anyone got any ideas ? or maybe someone tried this before ?
Cheers
PS;
Also, if this holds true, one could attempt the same reaction using Methane gas and generate TCM using Oxidation instead of free radical mechanism
|
|
|
Texium
|
Thread Moved
27-11-2017 at 08:59 |
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's possible, but you need to get it very hot
http://onlinelibrary.wiley.com/doi/10.1002/maco.19900410803/...
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
No. Chlorine is nowhere near as powerful an oxidizer as oxygen. The free-radical reaction is catalyzed by UV light, which breaks the chlorine
molecule's bond, producing monatomic chlorine. This species, then, has sufficient power to attack the carbon-hydrogen bond.
Think of a room full of natural gas and oxygen. Now, methane is, in fact, a very stable compound. But oxygen is actually one of the few reagents that
will attack it. Consider lighting a match in such a room. All that was needed was initiation energy.
Since we live in an oxygen-containing atmosphere, we often underestimate just how powerful a reagent oxygen really is.
Chlorine, bromine vapor, and maybe iodine vapor can do this reaction, but only with free radical initiation. To get sulfur vapor to do it, you need
great heat, like that of a coke oven, at which point you might as well just start with the carbon.
The free radical addition reaction wouldn't actually be so bad in a home lab environment, as you could condense out the CCl4 pretty readily, by
controlling the reaction vessel temp. All you have to do is make sure you are above the boiling point of chloroform (and DCM), but below that of CCl4,
to use the state change to drive the reaction forward. Eventually, you would have nothing but HCl gas and liquid CCl4. Hmm.
Maybe start out with chloroform vapor mixed with Cl2, exposed to a UV light? I'm thinking about how an apparatus might be set up...
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Further thought...
Cl-Cl bond energy is 243 kJ/mol, corresponding to photon energy of 490 nM. Pyrex is opaque to UV at this wavelength, so the UV source would have to be
inside the apparatus, or the reaction vessel would have to be made of a UV-transparent material. The polyacrylate material of UV-Vis cuvettes could
work, but is it resistant to Cl2, CHCl3, and CCl4?
The condensing and collection part could be away from the UV irradiation part, so it could be made of standard Pyrex.
Chloroform boils at 62, CCl4 at 76. Easy for a home lab to operate between those temps. Polyacrylate should hold up OK at those temps, too, if any
corrosion problem is addressed.
[Edited on 11/27/17 by PirateDocBrown]
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by BezeneBoy  | I hope this is the right thread;
I know that fluorine gas will spontaneously ignite carbon to form oxidation products that are Fluorocarbons,
Hence under the right conditions one could expect chlorine to oxidise carbon to Chlorocarbons, one of which, would be tetrachloromethane, a useful
solvent. I can't seem to find any resources on this as the production of TCM is performed using methane gas and the free radical mechanism.
I'd imagine it would require a very complicated setup, but I don't see any evidence pointing against the fact that chlorine will oxidise the carbon
much like oxygen does in regular combustion.
Anyone got any ideas ? or maybe someone tried this before ?
Cheers
PS;
Also, if this holds true, one could attempt the same reaction using Methane gas and generate TCM using Oxidation instead of free radical mechanism
|
I'd also like to point out that CCl4 is very toxic in general, most notably to the Liver. I'd avoid it if I were you!
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  |
Think of a room full of natural gas and oxygen. Now, methane is, in fact, a very stable compound. But oxygen is actually one of the few reagents that
will attack it. Consider lighting a match in such a room. |
Think of a room full of methane and chlorine; consider lighting a match in it.
The outcome would be fairly similar.
https://www.youtube.com/watch?v=nz03fpullyo
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Oh sure, there's a reaction, but the product is not chlorinated carbons, but simply soot. We want the reaction analogous to CH4 + 2O2 = CO2 + 2H2O,
i.e. CH4 + 4Cl2 = CCl4 + 4HCl. Instead we get CH4 + 2Cl2 = C + 4HCl.
Chlorine is simply unable to oxidize the carbon, even given the heat available from the HCl formation exotherm.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  | Further thought...
Cl-Cl bond energy is 243 kJ/mol, corresponding to photon energy of 490 nM. Pyrex is opaque to UV at this wavelength, so the UV source would have to be
inside the apparatus, or the reaction vessel would have to be made of a UV-transparent material. The polyacrylate material of UV-Vis cuvettes could
work, but is it resistant to Cl2, CHCl3, and CCl4?
The condensing and collection part could be away from the UV irradiation part, so it could be made of standard Pyrex.
Chloroform boils at 62, CCl4 at 76. Easy for a home lab to operate between those temps. Polyacrylate should hold up OK at those temps, too, if any
corrosion problem is addressed. |
Looked into quartz, and it seems transparent enough at the desired wavelength. Someone seeking to make any chlorinated alkane at home should be able
to pull this off, given a good UV source, and appropriate distillation gear to resolve the products. Hmm.
Since gone are the days when I could just flip open an Aldrich catalog and order any desired chloroalkane, this could be extremely useful, indeed. All
I need is a line on the right quartz tube and UV source.
Br-Br bond energy is 194 kJ/mol = 617nM. Also very doable.
Anyone else doing this sort of photochemistry?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Magpie did this, and posted the results here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=14...
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Marvelous! Of course I should have UTFSE.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Except that it does.
You need to get it hot.
I already posted a reference to prove this; why are you pretending it doesn't happen?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
https://en.wikipedia.org/wiki/Carbon_tetrachloride#History_a...
"Prior to the 1950s, carbon tetrachloride was, manufactured by the chlorination of carbon disulfide at 105 to 130 °C"
So first react the carbon with sulfur, then react the product with chlorine. Easy enough, right? But Tetra's warning about his
namesake compound ought to be heeded: unless you are both confident in your ability to handle CCl4 and sure that you need it, don't make it, it's very
toxic.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  | Further thought...
Cl-Cl bond energy is 243 kJ/mol, corresponding to photon energy of 490 nM. Pyrex is opaque to UV at this wavelength,...
[Edited on 11/27/17 by PirateDocBrown] |
490 nm is visible and pyrex glass is fairly transparent to visible light, in fact it's OK down to about 320nm, roughly where the Cl2 absorption max
is.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  |
Except that it does.
You need to get it hot.
I already posted a reference to prove this; why are you pretending it doesn't happen?
|
You are right, of course, it does happen at elevated temperature. I should have been more clear, and stressed that I meant at closer to ambient.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by PirateDocBrown  | Further thought...
Cl-Cl bond energy is 243 kJ/mol, corresponding to photon energy of 490 nM. Pyrex is opaque to UV at this wavelength,...
[Edited on 11/27/17 by PirateDocBrown] |
490 nm is visible and pyrex glass is fairly transparent to visible light, in fact it's OK down to about 320nm, roughly where the Cl2 absorption max
is. |
Hmm, did I get some bad data here? Or is my math wrong? Melgar cut away the glass cover of his Hg vapor light due to the absorption of hv at the
desired wavelength. Yet here is the published spectrum of borosilicate, supporting your persepective.
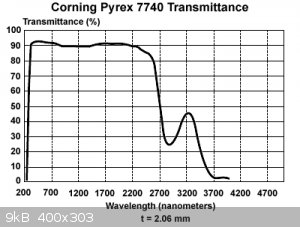
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Here it's compared to quartz.
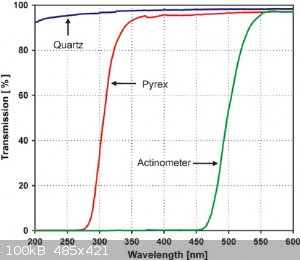
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by clearly_not_atara  | https://en.wikipedia.org/wiki/Carbon_tetrachloride#History_a...
"Prior to the 1950s, carbon tetrachloride was, manufactured by the chlorination of carbon disulfide at 105 to 130 °C"
So first react the carbon with sulfur, then react the product with chlorine. Easy enough, right? But Tetra's warning about his
namesake compound ought to be heeded: unless you are both confident in your ability to handle CCl4 and sure that you need it, don't make it, it's very
toxic. |
"namesake compound" ... *Hasn't even made or worked with CCl4* ... (cue Laugh track)
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
CCl4 used to be in every laundry room, as a spot remover.
It proved to be more toxic than was known, but that was mostly when kids got into it. Normal lab precautions, (gloves, ventilation, keeping reagents
in glass most of the time) make the danger pretty small.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If you use a low pressure Hg lamp nearly all the UV is at 254 nm- which is fine for a lot of photochemistry. It will, for example, split chlorine
However at that wavelength you do need quartz or something to transmit the UV. That's why a lot of photochemical kit was made from quartz
However with a medium pressure Hg lamp a lot of the UV is 366nm and Pyrex is fine (and cheap).
There's a couple of over the counter sources of quartz tube .
Spares for this sort of thing
https://www.amazon.co.uk/Quartz-Glass-Heater-Stainless-Styli...
and this sort
https://www.aliexpress.com/quartz-glass-aquarium_reviews.htm...
The aquarium stuff is interesting because, of course, they also sell UV lamps (though they are not very cheap)
Does anyone have experience of the UV lamps used to cure nail gels?
[Edited on 6-12-17 by unionised]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Yes, very hot. See the last method from Atomistry.com on CCl4 (link: http://carbon.atomistry.com/carbon_tetrachloride.html) to quote:
"Carbon tetrachloride, CCl4, is the final chlorination product of methane, but it is generally prepared by the action of chlorine on carbon disulphide
through the catalytic agency of a chlorine-carrier. Thus Hofmann dissolved antimony trichloride in carbon disulphide, and passed dry chlorine through
the solution. The trichloride became pentachloride, which then reacted with the carbon disulphide, thus:
CS2 + 2SbCl5 = CCl4 + 2SbCl3 + 2S.
Other catalysts which may be employed are chloride of bromine, chloride of iodine, phosphorus pentachloride, molybdenum pentachloride, and aluminium
chloride.
Carbon tetrachloride is also prepared in several ways by the action of chlorine on carbon in the electric furnace."
Now, while I do not have an electric furnace on hand, I do experiment with a microwave oven and have been impressed (more likely frightened) by plazma
arcs. Here is the comment from a source concerning, in particular, carbon materials:
"During the microwave heating process, graphite materials are reported to generate microplasma when they are heated in the presence of microwaves.
During this process, some of the electrons jump out with the increment of their kinetic energy, resulting in ionizing the surrounding atmosphere,
which is visualized as sparks of electric arc formation[4,5]"
Source: See https://www.researchgate.net/publication/207018862_Ball_ligh...
I would mention the temperature range of the plazma arc, but I have a hard time believing the magnitude of even the lowest cited temperature reading!
So applying a microwave pulse, one would think, to say graphite in an atmosphere of chlorine could meet, or possibly exceed, the temperature
requirement.
However, my personal experience with these arcs warns of extreme temperature inducing the cracking/rupture/burning of vessels. This suggests to me
that this small scale path is not likely to be successful, so avoid it and focus on other paths noted above.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PirateDocBrown  | No. Chlorine is nowhere near as powerful an oxidizer as oxygen. The free-radical reaction is catalyzed by UV light, which breaks the chlorine
molecule's bond, producing monatomic chlorine. This species, then, has sufficient power to attack the carbon-hydrogen bond.
Think of a room full of natural gas and oxygen. Now, methane is, in fact, a very stable compound. But oxygen is actually one of the few reagents that
will attack it. Consider lighting a match in such a room. All that was needed was initiation energy.
Since we live in an oxygen-containing atmosphere, we often underestimate just how powerful a reagent oxygen really is.
Chlorine, bromine vapor, and maybe iodine vapor can do this reaction, but only with free radical initiation. To get sulfur vapor to do it, you need
great heat, like that of a coke oven, at which point you might as well just start with the carbon.
The free radical addition reaction wouldn't actually be so bad in a home lab environment, as you could condense out the CCl4 pretty readily, by
controlling the reaction vessel temp. All you have to do is make sure you are above the boiling point of chloroform (and DCM), but below that of CCl4,
to use the state change to drive the reaction forward. Eventually, you would have nothing but HCl gas and liquid CCl4. Hmm.
Maybe start out with chloroform vapor mixed with Cl2, exposed to a UV light? I'm thinking about how an apparatus might be set up...
|
Per this demo https://www.youtube.com/watch?v=NN82GoBG98s the action of light results the propagation of an explosive chain reaction between Cl2 and H2. The
latter is initiated by blue light splitting the Cl2. The sequence is an initiation step followed by two propagation and three possible termination
steps:
INITIATION
Cl2 + UV = .Cl + .Cl
PROPAGATION REACTIONS
H2 + .Cl = .H + HCl
.H + Cl2 = .Cl + HCl
TERMINATION REACTIONS
.H + .H = H2
.Cl + .Cl = Cl2
.H + .Cl = HCl
Now, in the case of CHCl3, assuming chloroform itself is not easily light activated to produce radicals:
INITIATION
Cl2 + UV = .Cl + .Cl
PROPAGATION REACTIONS
CHCl3 + .Cl = .CCl3 + HCl
.CCl3 + Cl2 = .Cl + CCl4
TERMINATION
.CCl3 + .CCl3 = C2Cl6
.Cl + .Cl = Cl2
.CCl3 + .Cl = CCl4
Note, the same number of steps in both cases.
So, is it certain that the use of CHCl3 vapors with Cl2, in excess, does not actually result in an explosion with UV light? With air, CHCl3 is not
known to form explosive mixtures at normal atmospheric temperatures and pressures. But oxygen is not chlorine, and there may be a chain reaction
issue.
So perhaps this should be checked out on a small scale, and avoid any oxygen presence as it poisons the chain reaction for H2/Cl2/UV.
-----------------------------------
A note, direct sunlight acting on CH4 was confirmed by Mellor (see "Modern Inorganic Chemistry" page 693) with an excess of Cl2 to produce an
explosion hazard. This is actually interestingly as there are many more termination steps than propagation reactions in CH4/Cl2/direct sunlight.
However, the terminations that create new products could feed on an excess of chlorine (and chlorine radicals) leading to an explosive chain reaction,
as was claimed.
[Edited on 8-12-2017 by AJKOER]
|
|
|