| Pages:
1
2
3 |
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Second attempt at cobalt iodate:
I had another go at making cobalt iodate, basically trying the same double displacement reaction but at lower temperature. And I filtered the sodium
iodate solution before using.
- 5g NaIO3 in 30ml water, slight heat until all dissolved into a slightly brown colored solution.
- Filter the solution which cleared it up. Some dark specs as remainder; I don't know what this contaminant is, it does not appear to be free iodine.
- Dissolve 2.2g cobalt acetate in 12ml water, giving a red solution.
- Mixed the solutions as approximately 40C while stirring. The solution went through color changes as it cooled to room temp, see photos below. No I2
gassing off this time which was good!



- Vacuum filter, wash once with cold water in funnel, and keep sucking air through to dry it more. Color of this still moist product as below; bit of
a mix of colors.

- Leave out exposed to air in the shed to dry; after some 3 days (when I again remembered to look at it!) the color was as below, which to me is
lavender.

2.5g dry product recovered. I did 2 tests on the product which I think confirms it to be cobalt iodate:
- At high heat (on the center of the hotplate so should be around 300C) it decomposes. I2 fumes are seen and a black looking residue remains, probably
a cobalt oxide.
- It dissolves readily in concentrated hydrochloric acid, releasing bubbles that smells like Cl2 gas and forming a green solution.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making cobalt chloride CoCl2 anhydrous. My cobalt source is cobalt acetate - only because I got a reasonable quantity cheap! Seems that one
advantage of using cobalt acetate is that when acetic acid is a side product this is easy to boil out of solution. Cobalt chloride was made as
follows:
(CH3COO)2Co.4H2O + 2HCl = CoCl2 + 2CH3COOH + 4H2O
- 31g concentrated HCl added to a small beaker
- Slowly add cobalt acetate with stirring until a total of 30g added. This should have placed the HCl is excess.
- Leave stirring for a few hours. Very dark blue solution. Stop for evening, cover beaker with watch glass.
* Next day *
- Transfer very dark blue crystals and supernatant liquid to crucible and onto sand bath. Vinegar smell.
- Heat up on sand bath. Start to boil for while, with very strong vinegar fumes...had to open another door to get more draft through the shed. Boiling
at around 115C.
- Once getting near dry break up with nickel spatula while still being heated to prevent hard layers forming, easy to break up.
- Initially a dark blue mass. Now seems to be only steam coming off, vinegar smell much less.
- Color becoming lighter as it dries. See photo below showing the mix of some crystals still hydrated (the darker) and most already the final sky blue
color.

- Keep drying, check weight occasionally. Once all sky blue and weight seems constant (and close to the expected) dry for another 30 minutes and stop.
- Break into pieces small enough to go into the bottle and bottle while still very hot - compliments to whoever made these bottles, it did not crack!
- Final recovery was 15g, just half a gram or so less than the theoretical.
The leftover smears in the crucible quickly turned pink-red as it cooled down and started absorbing water. In the near future I want to try and make
the red hydrate.
Pictures of the hydrated salt below.


|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
May be my experience of getting a lower hydrate of CoCl2 could be relevant to this thread.
As you know there are blue anhydrous CoCl2, CoCl2 * 9 H2O which is violet with red tint as well as some intermediate hydrates also with some distinct
colours (I can attach photos later).
As a method to low a water content I found this is working well: CoCl2 * 9H2O is mixed with calculated amount of tert-butyl alcohol (tert-butyl
alcohol forms azeotrope with water) and then the alcohol & water is distilled of. As I can see as a result CoCl2 has a greater affinity to water
but there is some intermediate hydrate which can be formed by this method. So, this way I produced very nice crystals of intermediate hydrates.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Are you sure about that? I never heard about nonahydrate. Just about hexahydrate (red solid which crystallize out from CoCl2 solutions at room
temperature), dihydrate (violet solid, observable intermmediate during dehydratation of hexahydrate) and anhydrous (blue solid).
Lion850: It is easier to precipitate basic cobalt carbonate from cobalt acetate solution and dissolve it in hydrochloric acid. Than just evaporate
water and dehydrate red hexahydrate.
[Edited on 18-9-2020 by Bedlasky]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Bedlasky - I will try that. I am getting 500g basic cobalt carbonate; its waiting to be collected when I next go into town.
Teodor - I look forward to see the photos.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cobalt tungstate report: This was done in 2 stages:
2NaOH + H2WO4 = Na2WO4 + 2H2O
Na2WO4 + (CH3COO)2Co.4H2O = CoWO4 + 2CH3COONa + 4H2O
- 3.1g NaOH dissolved in 10g water. Clear solution.
- 9.8g H2WO4 was added in small quantities, waiting between additions until the yellow powder dissolved into the clear solution.
- The last H2WO4 added took some time to disappear, and the final solution had a very pale yellow tinge. See photo below.

- 9.4g cobalt acetate dissolved in 30g water, very dark red solution. See photo below.

- Add the sodium tungstate solution slowly to the cobalt acetate solution while stirring. Light purple colored suspension formed, photo below. Stir
for 10 minutes.

- Attempt to go a gravity filtration - very slow! Ran out of patience and transfer to vacuum filter.
- Purple remainder and light red filtrate, see photo below. Seems the cobalt acetate was in slight excess. Wash with water in filter twice, clear
run-through.

- Dry on steam bath. As the product dried the color lightened a bit (more distinct to the naked eye than on the photos).
- 11.8g of light purple of presumably cobalt tungstate recovered. Photo of final product below.

I searched online for the color of cobalt tungstate and various colors are mentioned, like red. However there are some photos that shows a similar
color to what I achieved, for example:
https://www.reddit.com/r/chemistry/comments/big18p/insoluble...
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Cool! I read in one book that CoWO4 is purple, but I remember, that when I mixed CoCl2 with Na2WO4 I obtained pink precipitate. I plan to do some W
chemistry and compare it with Mo chemistry. So I'll check this precipitation again.
[Edited on 22-9-2020 by Bedlasky]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making cobalt formate Co(HCO2)2
Cobalt formate was made by reacting formic acid (90% AR grade) with cobalt carbonate (from a ceramic supplier):
2CH2O2 + CoCO3 = Co(HCO2)2 + CO2 + H2O (although the final product was most likely the dihydrate).
- 9g of 90% formic acid in small beaker
- 10.7g of cobalt carbonate measured out, add 2 small portions.
- Not much of a reaction. Add 15ml water and gentle heat on hotplate.
- Reaction start when warm (beaker can still be held by fingers) with bubbling. As expected when reacting a carbonate and acid, but not as vigorous as
the common strong acids.
- Add all remaining CoCO3, stir and gentle heat. Solution color slowly changes to red.
- After the reaction seemed to have stopped add another 2g formic acid to ensure acid in slight excess.
- Keep stirring another 30 minutes, no more bubbles.
- Seems still to be some unreacted purple powder - cobalt carbonate? Transfer to bigger beaker, in 250ml water, bring to boil. Nearly all solids
dissolved into red solution.
- Gravity filter hot. Red filtrate, a very small amount of purple-ish remainder.
- Steam down solution slowly, crystals started to appear around 100ml point.
- Switch off hot plate and leave to cool down slowly overnight.
- Next day: lots of crystals in the cold solution (morning ambient in the shed around 18C). Decant the supernatant red liquid (this will be the second
crop) and transfer the red/maroon crystals to some paper towel and then a watch glass to dry. Crystals looks like little plates.
- Seems completely dry after a few hours. Picture of the dry product below.

- 9g recovered, 2nd crop still to be added.

When steaming down the beaker with the second crop, I got tied up and when I went back it was completely dry and it was turning a more purple color. I
did not know whether the purple was the anhydrous salt or whether it started to decompose; as the decomposition temp of cobalt formate is said to be
around 140C. I added 50ml water to the purple/red mix and all dissolved back into a red solution again, so it would seem the purple is the color of
the anhydrous salt or a lower hydrate.
So far this is the prettiest cobalt salt I have made.
[file]83372[/file]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  |
Are you sure about that? I never heard about nonahydrate. Just about hexahydrate (red solid which crystallize out from CoCl2 solutions at room
temperature), dihydrate (violet solid, observable intermmediate during dehydratation of hexahydrate) and anhydrous (blue solid).
[Edited on 18-9-2020 by Bedlasky] |
Probably you are right. So, I've made probably CoCl2 * 2H2O from CoCl2 * 6H2O by this method. I didn't check the water contents, it was just an
experiment to check how the alcohol acts on the hexahydrate (in some old children book I have an experiment of destroying CoCl2 * 6H2O by acetone, so
I just was experimenting with this approach). Here are the photos:
 
[Edited on 23-9-2020 by teodor]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Nice colors Teodor. I tried to make the hexahydrate but ended up with a mix of colors.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Atomistry says “Cobalt iodide is most readily obtained in solution by warming the finely divided metal with water and iodine” and this was my
preferred route….but I did not have cobalt powder. So I made cobalt powder by reducing cobalt sulphate with sodium borohydride:
CoSO4.7H2O + 2NaBH4 = Co + H2 + B2H6 + Na2SO4 + 7H2O
- 17.9g cobalt sulphate hydrate dissolved in 110g water
- 10g NaBH4 was added in VERY small portions, each time waiting for the fierce bubbling to die down before adding the next (mainly H2 coming off as
far as I know; looks good then set alight in the beaker). This is more or less double the stoichiometric amount, as seems to be recommended when I was
reading up on reducing metal compounds to the metal.
- The final solution was black and was vacuum filtered. The remainder was a dark grey / black powder which was rinsed in the funnel with lots of
water.
- The still-wet remainder was tested with concentrated hydrochloric acid. It very quickly dissolved with some bubbling into a light blue solution,
which turned maroon-ish when water was added. All the powder dissolved with nothing left over. So it seems it was mostly cobalt powder.
** I later saw that a very small amount of black ppt settled out of the filtrate once it cooled, and this black ppt did not seem to react with HCl. I
don’t know if this was maybe a boron compound.
- My aim was to react 10g of iodine to form cobalt iodide. The reduction of the cobalt sulphate should have yielded 4g metallic cobalt, which was way
in excess to react with 10g iodine.
- The rest of the wet powder was added to a beaker with 100g water, and 5 gram iodine crystals. After some time of stirring the iodine was gone and
the solution a dark red. 5 more grams of iodine was then added, giving a total of 10g.
- The iodine again seemed to react in some 10 minutes but the stirring was continued for another 2 hours (I got called away). The solution was then
gravity filtered to remove the excess cobalt.
- The filtrate was a red solution, see photo below.

- A few drops of this red solution was added to a lead nitrate solution, giving a bright yellow ppt of most likely lead iodide. See photo.

- The solution was boiled down. No I2 fumes were observed at boil which was seen to indicate there was not free iodine in the solution. Once the
volume was less than 50ml it was transferred hot to the desiccator, the still warm solution boiled a bit more as vacuum was applied.
- After some 24 hours there was a dark red-ish mass in the desiccator. Photo below.

- But when I looked again a few hours later it turned black/very dark green! Photo below.

- After a further 48 hours (total 72) in the desiccator the contents appeared solid from the outside and it was taken out. But it was still wet under
a crust, and once in air it started to fume gently and turned to green where it was thinly layered. Photo below, although it did not really capture
the fuming.

Atomistry do mention a black form of CoI2 that slowly turns green on exposure to air but it does not mention any fuming in air. The fumes don’t
ready have a smell, could be a hint of a smell similar to H2S.
- I then put it on a steam bath. It seemed to first melt to a black liquid and then form a crust on top which prevented further drying. Once this
crust was broken it fumed again.
- I decided that it cannot be easily dried in air. Online reading said cobalt iodide was soluble in acetone. I added acetone and got a dark green
solution.

- This solution was filtered, and there was no remainder. Dripping this solution into a lead nitrate solution again gave the bright yellow ppt of lead
iodide. The dark green filtrate was bottled and labelled “cobalt iodide in acetone”. See photos below.
 
Getting solid CoI2 out of a water solution probably needs working in an inert atmosphere?
As a side note, the cobalt remainder that was left when the iodine solution was filtered off has been exposed to air for some days now and is turning
olive green, see below. I don’t know which compound is forming?

|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cobalt monosulphide CoS
A double displacement reaction was performed between sodium sulphide solution and cobalt sulphate solution. A black ppt was immediately obtained.
After some 30 minutes of stirring the solution was vacuum filtered, and the remainder washed with water in the funnel. During all of this there was
only the occasional hint of H2S smell. Photo of the wet remainder below.
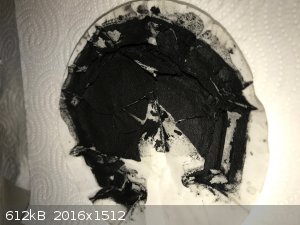
The remainder was dried on a steam bath, the dry product is more brown. Photo below. There was no smell while it dried.

The product does not readily dissolved in hydrochloric acid, but it does dissolved in dilute nitric acid. However, there is hardly any bubbles when it
dissolves in nitric acid and no smell of H2S.
Textbook of inorganic chemistry vol 9-1 says that CoS does not always react with HCl but does dissolves in HNO3; and colors can be black or brown. So
I think there is a reasonable chance I ended up with CoS (or another sulphide) but I'm not 100% sure.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Cobalt sulphate & cobalt iso-butyrate
As a beginner in the cobalt chemistry I've made just some cobalt sulphate. I put a piece of cobalt metal in diluted H2SO4 and found this after 3 days.

I had attempt to crystallise cobalt iso-butyrate but it doesn't want to crystallise at all. But I must confess my starting compounds were not pure.
[Edited on 2-11-2020 by teodor]
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Quote: Originally posted by teodor  | As a beginner in the cobalt chemistry I've made just some cobalt sulphate. I put a piece of cobalt metal in diluted H2SO4 and found this after 3 days.
I had attempt to crystallise cobalt iso-butyrate but it doesn't want to crystallise at all. But I must confess my starting compounds were not pure.
[Edited on 2-11-2020 by teodor] |
Very nice, the Cobalt Sulfate looks beautiful. Simple experiments are sometimes the best.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by MidLifeChemist  | Quote: Originally posted by teodor  | As a beginner in the cobalt chemistry I've made just some cobalt sulphate. I put a piece of cobalt metal in diluted H2SO4 and found this after 3 days.
[Edited on 2-11-2020 by teodor] |
Very nice, the Cobalt Sulfate looks beautiful. Simple experiments are sometimes the best. |
Thank you very much. But I believe it was a first step only - also to check what people can do with that 15% H2SO4 we will have in EU. For the next
step I have some Ba2S2O6 (barium dithionate). I'd like to make some cobalt dithionate. Don't ask me why. I have no idea yet but want to do some
experiments with it.
What is still completely unclear for me are the rules how cobalt makes complexes. About chromium I know we can do much during oxidation from (II) to
(III) state. But what is the general rule by which we can attach or replace ligands in a cobalt compound?
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Just mix some ordinary cobalt salt (chloride, sulfate, nitrate) with ligand. Ligand exchange in cobalt complexes is usually quick (unlike trivalent
chromium where ligand exchange is slow, this is reason why Cr(III) complexes are often make from Cr(II) or Cr(VI) compounds). Nitrates, perchlorates
and sulfates are weak ligands, so they can be exchanged by almost everything. Chlorides are stronger ligands, but they can be exchanged for wide range
of other ligands, especially organic ligands, CN-, F-, NH3, SCN- which forms quite strong complexes.
Other factor is constant of stability. The bigger constant is, the more is equilibrium shift to the right (so the more stable complex is). You can
find these constants online or in analytican tables for most common ions and ligands.
You can also look at HSAB theory.
[Edited on 2-11-2020 by Bedlasky]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Bedlasky  | | Just mix some ordinary cobalt salt (chloride, sulfate, nitrate) with ligand. Ligand exchange in cobalt complexes is usually quick (unlike trivalent
chromium where ligand exchange is slow, this is reason why Cr(III) complexes are often make from Cr(II) or Cr(VI) compounds). |
That's only for Co(II). Cobalt(III) is as slow as chromium(III). But that's generally only stable with N-donor ligands such as ammonia, en, or
nitrite.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I appreciate your answers.
I noticed that some (II) compounds of cobalt, like "simple" (H2O) salts (Cl-, SO4^2-, NO3-) are probably quite stable to aerial oxidation, but basic
forms / hydroxides as well as some complexes are not. Is it connected with the "stability" concept, so is it basically the same property as stability
of ligands or is it something different? Generally, should be oxidation of Co atom analysed with connection with ligands exchange or it is completely
unrelated process?
Edit: this relation is really strange for me, as for a beginner, because Co behaviour towards nitric acid is really unusual - HNO3 usually oxidises a
metal to higher valency, but it seams there is no Co(NO3)3 exists (with H2O ligands) but [Co(NH3)6](NO3)3 does exist.
[Edited on 3-11-2020 by teodor]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nitric acid will often bring metals to their higher oxidation state because it's a good oxidizing agent. However, no oxidizing agent is going to
bring cobalt to the +3 state in aqueous solution, because [Co(H2O)6]3+ will oxidize water.
Oxides and hydroxide will be more stable as higher oxidation states than the corresponding aqueous ions; ions like cobalt(II), iron(II) and
manganese(II) are perfectly stable in solution, but if you precipitate them as the hydroxides, they are very easily oxidized by the air. One way to
look at this is with le Chat's principle. Picture an equilibrium between the +2 ion and a mild oxidant on one side, and the +3/+4 ion on the other.
If you increase the pH, the more highly-charged ion will be more likely to hydrolyze and drop out of solution as the hydroxide. This causes the
reaction to shift to the products side to replace it, and soon all of your metal is present as the solid high-valent oxide or hydroxide.
Cobalt(III) is stabilized by good donor ligands- amines, usually, but also carbonate and nitrite will work. K3[Co(NO2)6] is very easy to make;
K3[Co(CO3)3] is a little more challenging.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by teodor  |
Edit: this relation is really strange for me, as for a beginner, because Co behaviour towards nitric acid is really unusual - HNO3 usually oxidises a
metal to higher valency, but it seams there is no Co(NO3)3 exists (with H2O ligands) but [Co(NH3)6](NO3)3 does exist. |
This isn't necessary true. Look at Mn, Ni, Cu, Tl or Pb. Or even Hg, which is oxidized by dilute nitric acid to (Hg2)2+ (by concentrated to Hg2+).
Co(III) is strong oxidizing agent which oxidize water, this is why nitric acid oxidize cobalt just to II oxidation state. These ligands like ammonia,
edta, cyanide etc. stabilise Co(III) but I don't know why. Hexacyanocobaltate(II) is even oxidized by water to hexacyanocobaltate(III).
[Edited on 3-11-2020 by Bedlasky]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Bedlasky  | | These ligands like ammonia, edta, cyanide etc. stabilise Co(III) but I don't know why. Hexacyanocobaltate(II) is even oxidized by water to
hexacyanocobaltate(III). |
IIRC, it's stabilized by strong-field ligands, which increase the energy difference between the three stabilized d orbitals and the two destabilized d
orbitals. Cobalt(II) is d7, so it's got six electrons in the three very stable orbitals, and one in a destabilized orbital. The stronger the
splitting from the ligands, the less stable (and more easily removed) that last electron is. Water, not so much. Ammonia is a stronger ligand, and
cyanide is one of the strongest.
(I may not have all of the terms exactly right- I haven't done anything with crystal field theory in decades, but that's the gist.)
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Quote: Originally posted by Lion850  |
** I later saw that a very small amount of black ppt settled out of the filtrate once it cooled, and this black ppt did not seem to react with HCl. I
don’t know if this was maybe a boron compound.
|
When I do Cobalt chemistry, I often get small amounts of a black powder that doesn't react with HCl, or reacts very very slowly. I'm pretty sure it is
Co2O3 or Co3O4. But not sure in your case.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
@Bedlasky @DraconicAcid Thanks!
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cobalt phosphate:
I first tried to dissolve cobalt carbonate in phosphoric acid (which worked so well with copper carbonate) but it became a sticky mess. I then tried a
double displacement reaction. The only soluble phosphate salt I had was potassium dihydrogen phosphate, KH2PO4.
- 15.7g of KH2PO4 dissolved in 70ml water, with some heat and magnetic stirring soon gave a clear solution.
- 14g cobalt acetate Co(CH3COO)2.4H2O dissolved in 40ml water, all dissolved into a dark red-purple solution.
- Add the cobalt acetate solution slowly to the potassium dihydrogen phosphate while stirring. Immediately got a lavender solution. Continued to stir
for 15 minutes. Photo below.

- Vacuum filter. Filtrate a very pale red. Rinse in funnel with 2 portions of water. Remainder a mix of lavender and darker purple, photo below.

- Leave on bench overnight. The next day the weight had reduced by 3g and the color was more uniform. Photo below.

- Dry on steam bath for many hours until weight stopped reducing: from 52g to final weight of 12.1g.
- Powdered in mortar, difficult as the product is quite hard.
- 11.8g dry lavender powder bottled.

Question: What is the correct equation for this reaction? Is it
2KH2PO4 + Co(CH3COO)2.4H2O = 2KCH3COO + Co(H2PO4)2 + 4H2O
but does Co(H2PO4)2 even exist?
Once other observation: cleaning the equipment afterwards showed the product dissolved easily in concentrated HCl giving a lovely blue solution, most
probably CoCl2.
-
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Interesting result. I made dark violet to blue hydrated cobalt phosphate from K2HPO4 and CoCl2.2H2O. I also made lovely magenta NH4CoPO4.H2O from
NH4Cl, K2HPO4 and CoCl2.2H2O. Maybe you make some double phosphate? KH2PO4 forms more acidic solution, maybe thats why I made different phosphate from
K2HPO4.
Btw. Atomistry also mention CoHPO4, but they say nothing about colour.
[Edited on 28-11-2020 by Bedlasky]
|
|
|
| Pages:
1
2
3 |