| Pages:
1
2 |
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@ Loptr, may be you achieve what you are looking for following this way. If so, you deserve an article at chemical periodic bulletins, cause to reduce
the double bond of insatured carbonyl compounds without affect the carbonyl group deserve a honor medal. It would solve a lot of pharmacological
synthesis problems.
Post your results here. Of course all SM comunity is anxiously waiting for your good results.
[Edited on 1-8-2018 by Chemi Pharma]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Loptr: any word on dithionite?
According to this paper:
https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1097-4660(199807)72:3%3C264::AID-JCTB897%3E3.0.CO;2-2
acetal formation occurs spontaneously during the hydrogenation of cinnamaldehyde in alcohols. Taking this as a guide, it seems like a good idea to try
the hydrogenation in ethylene glycol.
| Quote: | | According what I have studied, It's not possible to reduce the double bond of cinnamaldehyde without reduce the carbonyl group either.
|
Of course this is very silly, anyone who is familiar with the literature on these compounds knows that catalytic hydrogenation tends to attack the C=C
double bond whereas hydride reagents attack the carbonyl. It's not surprising that someone who only pays attention to hydrides would be misled.
However, even rudimentary searches turn up many systems with good selectivity for the conversion of cinnamaldehyde to hydrocinnamaldehyde; the
difficulty is that many involve rare metal catalysts on exotic supports, although you occasionally see methods using eg nickel phosphide for this
transformation.
https://www.sciencedirect.com/science/article/pii/S0926860X0...
https://www.sciencedirect.com/science/article/pii/S138111690...
https://www.sciencedirect.com/science/article/pii/S0926860X9...
https://www.sciencedirect.com/science/article/pii/S016943320...
https://link.springer.com/article/10.1007/s10562-008-9472-y
Impossible, indeed...
[Edited on 1-8-2018 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
oh, clearly_not_atara, you are so smart!
If I were not a man I will fall in love with you! argh!
Yes, I'm involved with borohydrides complex in my recent researchs. Any problem with this? Any problem if I don't know about anything despite you
PROFESSOR?
The problem here is you man! You claims I don't look about the real necessity of SM members, but you, in all your posts you preach the use of exothic
reagents, hyper complexes techniques and think all of this is normal an acessible for the amateur chemistry.
You complain about me wanting to favour a healthy competition with other SM suppliers to put down the chemical prices in bennefit of all SM members,
but YOU, is the first that post about reactions that use exothic and inacessible reagents to the home user. Tell me what are you really intend to do
here? joke with our faces? Or prove that you are more inteligent than the others?
Stop to criticize other members and try to be more polite here. I'm fed up your rudeness. Your ironies are deserving a moderator action!
I have no problems with @Loptr anymore after we talked at U2U. The only one I still having problems here is YOU! Come on man, call me at U2U and tell
me what's your real problem with me. Until there, don't piss me off, ok?
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
No, this is that time I spoke of that I would be able to start experimenting. I have a couple reactions planned, and the dithionite/PTC is one of
them.
Planned is a strong word. I am gathering the info to produce a planned reaction. 
Any ideas on tests and work up would be great. Solvents, tests, etc.
[Edited on 1-8-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by clearly_not_atara  | According to this paper:
https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1097-4660(199807)72:3%3C264::AID-JCTB897%3E3.0.CO;2-2
acetal formation occurs spontaneously during the hydrogenation of cinnamaldehyde in alcohols. Taking this as a guide, it seems like a good idea to try
the hydrogenation in ethylene glycol.
|
I had thought about the use of acetalation in an attempt to protect the carbonyl from reduction, but thought the double bond was then isolated as is
the case with styrene derivatives. They can be reduced, but under more strenuous conditions. The article mentions something like ~150 PSI of hydrogen.
What do you think about this under CTH conditions, or 1 atm.?
I was planning on purchasing some ethylene glycol soon for protection of ketones. 
[Edited on 1-8-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Chemi Pharma: I replied specifically to your statement that the reduction is "not possible" and would constitute a breakthrough in organic chemistry
if it were to be achieved. These claims are baseless, and I provided examples from the literature to demonstrate this. I never suggested the reactions
in those links were practical for amateurs, because that's not what I was talking about.
In fact those links are just the first five Google Scholar results for the query "cinnamaldehyde hydrocinnamaldehyde". A review states:
https://www.tandfonline.com/doi/abs/10.1080/0161494980800710...
"The hydrogenation of alpha,beta-unsaturated carbonyls into saturated carbonyls is comparatively easy to achieve because thermodynamics favor the
hydrogenation of the C=C bonds."
Loptr: Transfer hydrogenations are rare here. Metal-free reductions based on the Hantzsch ester look interesting. One version uses Hantzsch ester with
dibenzylamine:
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.200461...
Others exist as well:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.2004624...
There are some others using isopropanol and complex catalysts, but I'm guessing that standard Pd/C/formate will give okaaayyyyyy
regioselectivity with the major source of difficulty being the separation of hydrocinnamaldehyde from the unreacted starting material. One advantage
of transfer hydrogenation is the ability to control the quantity of reductant, which may prevent over-reduction.
For transfer hydrogenation of an unactivated double bond with Pd/C and ammonium formate, see:
https://www.sciencedirect.com/science/article/pii/S004040390...
I am slightly concerned that the use of ammonium formate with cinnamaldehyde may result in reductive amination or Mannich reactions. Perhaps a
tertiary ammonium formate (eg triethylammonium) would be better.
[Edited on 1-8-2018 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I just have a hard time spending so much for so little Pd/C. Maybe I will just buy a Pd bar, and then dissolve that in aqua regia. I have seen papers
on preparing the Pd/C catalysts, but can't recall how easy it is. It was something I perused when reading up on supported catalysis.
$316.61 - 10g Pd
https://www.apmex.com/product/96681/10-gram-palladium-bar-pa...
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You know the Hantzsch ester/dibenzylamine method is metal-free, right? I don't know what Hantzsch ester costs but I'm betting that ethyl acetoacetate,
formaldehyde and ammonia are all pretty cheap. Dibenzylammonium trifluoroacetate might be a little more expensive... but not at 5% catalyst loading.
I'm not sure how to make dibenzylamine, but I don't imagine it being very hard. At the least, a mixture of mono/di/tribenzylamines will be easy to
separate by distillation.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Reference 42 (equation 28) looks very possible, simple and OTC........
http://reag.paperplane.io/00003049.htm
There is also a REAXYS upload in Reaxys thread of all possible routes to hydrocinnamaldehyde.
I'm sure there are a few more reactions using cinnamaldehyde as a substrate.
And a few more that haven't even been tried yet.
/CJ
[Edited on 2-8-2018 by Corrosive Joeseph]
Attachment: Chapter 18 - Reduction of a,b Unsaturated Carbonyl Compounds.pdf (4.4MB)
This file has been downloaded 695 times
[Edited on 2-8-2018 by Corrosive Joeseph]
Attachment: Partial Reduction of Enones, Styrenes and Related Systems.pdf (7.8MB)
This file has been downloaded 618 times
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Loptr  |
No, this is that time I spoke of that I would be able to start experimenting. I have a couple reactions planned, and the dithionite/PTC is one of
them.
Planned is a strong word. I am gathering the info to produce a planned reaction. 
Any ideas on tests and work up would be great. Solvents, tests, etc.
[Edited on 1-8-2018 by Loptr] |
I found an article that reduces chalcones under dithionite/PTC conditions in CH2Cl2 with TBAHS.
Attachment: 12_chapter 5.pdf (1.1MB)
This file has been downloaded 430 times
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
The paper known as 'camps1986' AKA ' Regiospecific Reduction of Unsaturated Conjugated Ketones with Sodium Dithionite under PTC',
kindly uploaded by clearly_not is by far the most attractive looking dithionite method IMO.
If I was to attempt it I would try a two-phase toluene/water reaction with 'biocide' PTC......... 
https://www.bonnymans.co.uk/products/product.php?categoryID=...
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Although you guys are bravely looking for a decent way to reduce cinnamaldehyde to hydrocynnamaldehyde, I'm no convinced yet about this possibility
and really want that who can do it in practice, post the results here.
Just a note for the one who understands: I never said it's not possible to reduce the double bond without reduce the carbonyl group, I just have said
I humbly admit I don't know how to do it, despite my researchs, cause nobody knows everything, isn't right?
Every paper posted here, until now, tells about the reducion of cinnamaldehyde to hydrocinnamyl alcohol. I want to see a kind of hydrogenation of
double bond that doesn't reduce the carbonyl group to an alcohol or alkane either.
Sorry, but dithionite wouldn't make this job, according with all that I have read here and the papers submited. The aldehyde will be reduced to an
alcohol either, according this papers.
@C/J brought an interesting paper dealing with zinc/cooper reduction of double bond, but the case he brought is about an amide, not an aldehyde,
extremely reactive.
Come on guys, bring something really effective to do that and prove it's effectiveness. It will be a great feat, I guess!
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Chemi Pharma  | Although you guys are bravely looking for a decent way to reduce cinnamaldehyde to hydrocynnamaldehyde, I'm no convinced yet about this possibility
and really want that who can do it in practice, post the results here.
Just a note for the one who understands: I never said it's not possible to reduce the double bond without reduce the carbonyl group, I just have said
I humbly admit I don't know how to do it, despite my researchs, cause nobody knows everything, isn't right?
Every paper posted here, until now, tells about the reducion of cinnamaldehyde to hydrocinnamyl alcohol. I want to see a kind of hydrogenation of
double bond that doesn't reduce the carbonyl group to an alcohol or alkane either.
Sorry, but dithionite wouldn't make this job, according with all that I have read here and the papers submited. The aldehyde will be reduced to an
alcohol either, according this papers.
@C/J brought an interesting paper dealing with zinc/cooper reduction of double bond, but the case he brought is about an amide, not an aldehyde,
extremely reactive.
Come on guys, bring something really effective to do that and prove it's effectiveness. It will be a great feat, I guess! |
I will have some time this weekend for a first experiment. I will post my results and get feedback. I will test for double bonds and alcohols after
the reaction. I plan to precipitate any remaining aldehyde from the solution using the bisulfite adduct, back extract, and then test for alcohols.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Quote: Originally posted by Chemi Pharma  |
@C/J brought an interesting paper dealing with zinc/cooper reduction of double bond, but the case he brought is about an amide, not an aldehyde,
extremely reactive.
|
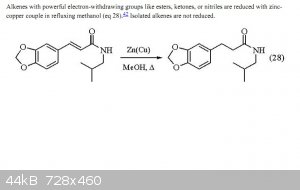
It takes out double bonds conjugated with carbonyls..........
"Alkenes with powerful electron-withdrawing groups like esters, ketones, or nitriles are reduced with zinc-copper couple in refluxing methanol"
(eq 28) [Ref. 42]
More later.
/CJ
[Edited on 2-8-2018 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@/CJ, the paper you brought, deal about esters, ketones and nitriles. And the example given, was about an amide. I doubt high reactive aldehyde group
woudn't be reduced to an alcohool as well.
Prove me I'm wrong, ok?
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
@ Chemi Pharma - You might be interested to know the nitro group is also a polar EWG.......
Back on topic.......... This deserves to be here............ Many, many ways...... But not really
/CJ
Attachment: 3-Phenylpropanal.pdf (3.3MB)
This file has been downloaded 878 times
[Edited on 3-8-2018 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Loptr  | Quote: Originally posted by Chemi Pharma  | Although you guys are bravely looking for a decent way to reduce cinnamaldehyde to hydrocynnamaldehyde, I'm no convinced yet about this possibility
and really want that who can do it in practice, post the results here.
Just a note for the one who understands: I never said it's not possible to reduce the double bond without reduce the carbonyl group, I just have said
I humbly admit I don't know how to do it, despite my researchs, cause nobody knows everything, isn't right?
Every paper posted here, until now, tells about the reducion of cinnamaldehyde to hydrocinnamyl alcohol. I want to see a kind of hydrogenation of
double bond that doesn't reduce the carbonyl group to an alcohol or alkane either.
Sorry, but dithionite wouldn't make this job, according with all that I have read here and the papers submited. The aldehyde will be reduced to an
alcohol either, according this papers.
@C/J brought an interesting paper dealing with zinc/cooper reduction of double bond, but the case he brought is about an amide, not an aldehyde,
extremely reactive.
Come on guys, bring something really effective to do that and prove it's effectiveness. It will be a great feat, I guess! |
I will have some time this weekend for a first experiment. I will post my results and get feedback. I will test for double bonds and alcohols after
the reaction. I plan to precipitate any remaining aldehyde from the solution using the bisulfite adduct, back extract, and then test for alcohols.
|
So how should I test for the presence of that double bond if the aldehyde is in fact not touched? Should I oxidize the aldehyde to the carboxylic acid
using sodium chlorite, and then use KMnO4 to test for the double bond?
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | So how should I test for the presence of that double bond if the aldehyde is in fact not touched? Should I oxidize the aldehyde to the carboxylic acid
using sodium chlorite, and then use KMnO4 to test for the double bond? |
According to this:
https://pubchem.ncbi.nlm.nih.gov/compound/3-Phenylpropanal#s...
3-phenylpropanal melts at 47 C, while cinnamaldehyde melts at -5 C:
https://en.wikipedia.org/wiki/Cinnamaldehyde
To be honest I find this a bit suspicious because cinnamaldehyde seems like it should be more polar and have a higher mp, but anyway, those are the
numbers, so that should serve to differentiate the compounds.
You should do OK with dithionite as long as you control the stoichiometry. Dithionite does reduce carbonyl groups, but it will generally reduce
alkenes first.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by clearly_not_atara  | | Quote: | | So how should I test for the presence of that double bond if the aldehyde is in fact not touched? Should I oxidize the aldehyde to the carboxylic acid
using sodium chlorite, and then use KMnO4 to test for the double bond? |
According to this:
https://pubchem.ncbi.nlm.nih.gov/compound/3-Phenylpropanal#s...
3-phenylpropanal melts at 47 C, while cinnamaldehyde melts at -5 C:
https://en.wikipedia.org/wiki/Cinnamaldehyde
To be honest I find this a bit suspicious because cinnamaldehyde seems like it should be more polar and have a higher mp, but anyway, those are the
numbers, so that should serve to differentiate the compounds.
You should do OK with dithionite as long as you control the stoichiometry. Dithionite does reduce carbonyl groups, but it will generally reduce
alkenes first. |
Ahh, so I could also attempt to separate them via distillation under vacuum. Maybe first try and see if I can separate them with partial
crystallization.
3-phenylpropanal, BP 217.7*C
cinnamaldehyde, BP 248*C
[Edited on 2-8-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That surprises me, because 3-phenylpropanoic acid has a melting point of 47 oC.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Loptr  | Quote: Originally posted by clearly_not_atara  | | Quote: | | So how should I test for the presence of that double bond if the aldehyde is in fact not touched? Should I oxidize the aldehyde to the carboxylic acid
using sodium chlorite, and then use KMnO4 to test for the double bond? |
According to this:
https://pubchem.ncbi.nlm.nih.gov/compound/3-Phenylpropanal#s...
3-phenylpropanal melts at 47 C, while cinnamaldehyde melts at -5 C:
https://en.wikipedia.org/wiki/Cinnamaldehyde
To be honest I find this a bit suspicious because cinnamaldehyde seems like it should be more polar and have a higher mp, but anyway, those are the
numbers, so that should serve to differentiate the compounds.
You should do OK with dithionite as long as you control the stoichiometry. Dithionite does reduce carbonyl groups, but it will generally reduce
alkenes first. |
Ahh, so I could also attempt to separate them via distillation under vacuum. Maybe first try and see if I can separate them with partial
crystallization.
3-phenylpropanal, BP 217.7*C
cinnamaldehyde, BP 248*C
[Edited on 2-8-2018 by Loptr] |
I will follow the following procedure taken from the camps1986 paper, with the exception of using toluene instead benzene. It has a higher boiling
point, so I hope it won't make too much of a difference. There is an azeotrope that should help with reflux.
I will separate the product mixture using fractional vacuum distillation separate the products. From the suspected aldehydes recovered via
distillation, I will make the oximes and check their melting points. CJ previously posted a paper regarding the reduction of cinnamaldehyde over
alumina that has a nice description of separating the two via vacuum distillation.


[Edited on 2-8-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Aha! That must be the
issue. Alfa Aesar shows a much more believable melting point of -42 C (and bp 222 C) for hydrocinnamaldehyde:
https://www.alfa.com/en/catalog/A10367/
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
| Pages:
1
2 |