Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Thionyl Chloride
November 30, 2017
A. Introduction
This preparation will follow that in Len1’s book (ref1) at ≈ 0.6 scale.
2SCl2 + 4SO3 + H2O --→ SOCl2 + 2HSO3Cl + 3SO2 (eqn 1)
B. Reagents
139.2g 65% oleum
132.1g SCl2
7.7g quinoline
15g boiled linseed oil
CaCl2
C. Apparatus Set-Up
1. Set up a 2-neck 1-liter rbf in an ice-water bowl over a magnetic stirrer-hotplate.
2. In the side-neck install an ice-water cooled condenser. Attach a CaCl2 guard tube to the outlet of the condenser.
3. Install a 125mL p-e funnel in the center neck.
4. Add a 3cm oval Teflon stir bar to the 1-liter rbf.

Photo1: thionyl chloride reaction vessel
D. Procedure
a. Reaction
1. Place 132.1g SCl2 in the rbf.
2. Place 139g of 65% oleum in the p-e funnel.
3. Drip oleum into the SCl2 at a rate of 1-3 drops/s with rapid stirring.
4. After all of the oleum has been added (about 20 minutes) the water bath temperature is raised to 75°C in the course of about 40 minutes.
5. After a final temperature of 75°C is reached, gentle reflux (1-2d/s) is continued for about 30 minutes to remove as much excess SCl2 and SO2Cl2
as possible from the mixture (no further SO3 is evolved beyond about 50°C).
Note: The paragraphs shown below in italics are excerpts from Len1’s book that are provided for explanation and are not part of the procedure
per se:
The reaction should not be continued further, since although SO2 evolution does not diminish, at this stage this is due to the decomposition of SOCl2
by reflux rather than formation of the product by the above reaction. The flask contents weigh 197g at this stage, corresponding to a weight loss of
80g due mainly to evolved SO2 and excess SCl2, as well as SOCl2 lost by decomposition.
b. Separation
1. The product is now transferred to a 500ml rbf and arranged for fractional distillation through an ice-water cooled condenser.
2. Discard the first few drops of SO3 and SCl2 then collect a low-boiling fraction boiling between 70°C–80°C. Filter it through a cotton plug.
(See Discussion below.)
3. Place the product left in the pot in a 100mL rbf and subject it to simple distillation. Collect the liquid condensing at 145°C-152°C.

Photo 2: fractional distillation for SOCl2 and HSO3Cl

Photo 3: simple distillation for HSO3Cl
The low-boiling fraction yields 81.7g, corresponding to crude SOCl2, containing a small amount of SO3, SO2Cl2, and SCl2/S2Cl2 impurity, while the
high-boiling fraction yields 46.2g, corresponding to an 80:20 mixture of HSO3Cl and S2O5Cl2. 26.5f 10% oleum (bp 175°C) remains in the flask, with
41g lost to gaseous products in the fractional distillation.
The combined yield of SOCl2/HSO3Cl/S2O5Cl2 with respect to free SO3, via the following reactions, is therefore 11.2%:
SCl2 + 4SO3 --→ SOCl2 + 3SO2 (eqn 2)
SOCl2 + 2SO3 --→ S2O5Cl2 + SO2 (eqn 3)
SCl2 + 3SO3 + H2O --→ 2HSO3Cl + 2SO2 (eqn 4)
This figure is explained by the fact that the residue of 10% oleum contains 18.2g ess SO3 than in H2SO4 content of the original oleum. This signifies
that more than the free SO3 content of oleum is active in the present reaction, and explains the high yield with respect to free SO3. It is
nevertheless appropriate to quote the yield on this basis because alternative preparations of the foregoing products operate on a free SO3 basis.
The gaseous weight loss in the course of the reactions amounts 118.8 of this 70.8g is accounted for by SO2 released in proportion to the products
collected from the reactions shown. A calculation shows that the remaining weight loss is made up of a volatilized 42g SCl2 and 7.7g SO3 either free
or in the form of the products of the reaction shown in eqn 1.
I transferred about 15mL of the residue remaining after the 1st cut was removed to a 25mL rbf and set up for simple distillation to recover the
chlorosufonic acid. Len1 says to take a cut that boils between 145°C and 152°C. I took the vigorously boiling liquid up to >200°C yet received
no condensate, and therefore no chlorosulfonic acid..
C. Purification
Purification Using Quinoline
1. Place 20.9g of SOCl2 in a 50mL rbf with Claisen adapter.
2. Connect the rbf to a distillation set-up using a condenser on ice-water protected from the atmosphere with a CaCl2 guard tube.
3. Add dropwise, with stirring, 2.5g of quinoline. The reaction generates considerable heat causing some of the SOCl2 to boil.
4. When all the quinoline has been added thermally insulate the flask and stillhead.
5. Slowly distill the SOCl2 (bp 75°C-77°C) until no more product comes over in this temperature range.
6. 54.4g of product is collected. About 6% of the thionyl chloride product is lost to decomposition.

Photo 4: purification of SOCl2 with quinoline
(See Discussion below.)
Purification with Boiled Linseed Oil
I did not perform this purification step as the loss in yield was too great for my small yield from the quinoline ourification. It is included here
for completeness.
1. The distillation flask is now replaced by the receiver.
2. Add 15g of boiled linseed oil.
3. Distill over the thionyl chloride as before.
42.0g of colorless liquid boiling in the range 77°C-78°C is collected representing an efficiency of 67% for the linseed oil step and 53% for the
purification overall.
(See Discussion below.)
E. Results
thionyl chloride yield =10.2g; chlorosufonic acid yield = 0
expected yields: 65.3g (72%) for thionyl chloride; 37.7g chlorosulfonic acid
F. Discussion
The low yield of SOCl2 is extremely disappointing. It seemed as though everything was being converted to SO2 and elemental sulfur. I have no
explanation for this. See the SO2 spewing out the vacuum receiver tublature in photo 2 above.
G. Reference
1. Small-Scale Syntheses of Laboratory Reagents, 2011, by Leonid Lerner, p.194, CRC Press
Comments, questions, and suggestions are welcomed.
Attachment: phpoH3soW (158kB)
This file has been downloaded 819 times
[Edited on 1-12-2017 by Magpie]
[Edited on 1-12-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That is 10.2g more thionyl chloride than I have, and I think it's awesome. I really have no idea what the issue might be, but I wonder about the
possibility that the SCl2 was not fully chlorinated.
Edit: Bah, typo. I thought I had fixed that already....
[Edited on 1-12-2017 by JJay]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks JJay. If I can figure out what went wrong I will try this again.
SCl3 is not produced, but SCl2. This is made from S2Cl2.
I know that any contamination with water will cause a loss, mole per mole. I tried to be really careful about this.
I should check my oleum strength. It was 75% by freezing point. I can check it by titration.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Great work yet again Magpie. Inspiring stuff.
2 SCl2 + 4 SO3 + H2O => SOCl2 + 2 HSO3Cl + 3 SO3
Presumably this is the overall reaction, seeing as water in there will react with either of the other two reagents to make less exotic
species, like S, HCl and H2SO4, so perhaps water is the reason for the unexpected yield.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, that reaction is composed of two reactions, each happening in a separate pot. I combined them for brevity.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I wonder where all of the electrons came from; if sulfur was produced, something else had to be oxidized, right? Is it possible your SCl2 had degassed
some chlorine before you used it for this rxn? That should explain the production of sulfur.
Apparently ClSO3H reacts with oleum to make chlorosulfonic anhydride. I don't know the bp of (ClSO2)2O but I think it could certainly be above 200 C.
Assuming that this compound accounts for the missing chlorosulfuric acid, you should be able to recover chlorosulfuric acid by gassing with HCl, by
the rxn (ClSO2)2O + HCl >> SO2Cl2 + ClSO3H. Sulfuryl chloride is known to be inert to these conditions (woelen iirc did a long-term experiment
on this) and nonpolar so it will probably separate from the rxn mixture and could be removed by decanting.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I am fortunate to have a friend with access to NMR, GC-MS, and FTIR who ran my samples of thionyl chloride and quinoline. The FTIR for thionyl
chloride came out quite good. That for the quinoline was only a 59% match. I will post pictures of these spectra if I can get the quality good
enough.
Here are the spectra. The BIO-RAD scan for thionyl chloride is from Pub-Chem.
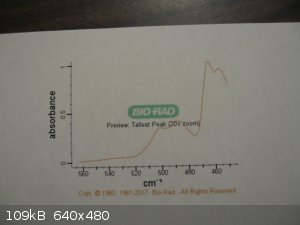
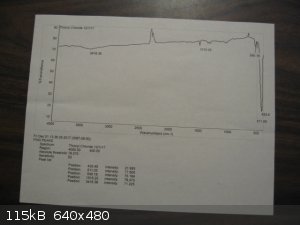 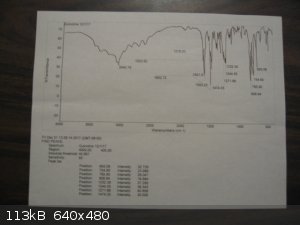
[Edited on 3-12-2017 by Magpie]
[Edited on 3-12-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The previous post shows the spectra for quinoline and thionyl chloride.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Hm. If the quinoline was partially reduced or contained some aniline, maybe it could have acted as a reducing agent? That would explain the sulfur.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Note that Brauer calls for 65% oleum, but it's for distilling SO3 into the SCl2.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here is an interesting chemically simple (but not likely simple in execution) direct path from atomistry.com to quote:
"Thionyl chloride is also formed by the oxidation with chlorine monoxide of sulphur in carbon disulphide or even of carbon disulphide itself"
Source: http://sulphur.atomistry.com/thionyl_chloride.html
Also page 267 in THE PRINCIPLES OF CHEMISTRY by D. MENDELEEFF available at https://books.google.com/books?id=CGwpbi0A060C&pg=PA267&... which also suggests S2Cl2 in place of CS2. To quote:
"Wurtz obtained the same substance by passing a stream of chlorine oxide through a cold solution of sulphur in sulphur chloride; the chlorine oxide
then combined directly with the sulphur, S + Cl20=SOCl2, whilst the sulphur chloride remained unchanged (sulphur cannot be combined directly with
chlorine oxide, as an explosion takes place). "
Note, to repeat, the direct action of sulfur on Cl2O is explosive!
Cited reaction is:
S + Cl2O = SOCl2 (in cold CS2 or S2Cl2)
Also, per Mellor on SOCl2 , page 424 (link: https://books.google.com/books?pg=PA424&lpg=PA424&dq... ), to quote:
"Thionyl chloride is also made by the action of chlorine monoxide upon sulphur at a low temperature, — 12°, to prevent explosion. It is also made
by adding sulphur trioxide to sulphur monochloride."
My suggested path to Cl2O is the action of CO2 on moist Ca(OCl)2 followed by drying of vapors. Namely:
Ca(OCl)2 + CO2 + H2O = CaCO3 (s) + 2 HOCl
2 HOCl = H2O + Cl2O
which I would describe as the formation and dehydration of highly unstable concentrated hypochlorous acid. I would recommend an excess of CO2,
diluting the Cl2O, as is done with the preparation of ClO2 from the action of an excess of oxalic acid (the source of CO2) acting on a chlorate.
An alternate water free path to Cl2O per Wikipedia is:
2 Cl2 + Na2CO3 → Cl2O + CO2 + 2 NaCl. (150-250 C)
Link: https://en.m.wikipedia.org/wiki/Dichlorine_monoxide
---------------------------------------------
Per Watts, a curious lesser known path to SOCl2 is claimed to proceed by the action of SO2 on SCl4, the latter only existing at low temperatures. To
quote Watts on the latter (link: https://books.google.com/books?pg=PA617&lpg=PA617&dq...
"SULPHUR TETRACHLORIDE SCl4. This compound exists only at temperatures under -20°. The molecular weight is probably 173.46 (SCl4).
Preparation.—S2Cl2 is cooled to below —20° (c. – 22°) and a slow stream of dry Cl is passed in until absorption of Cl ceases. Michaelis a.
Schifferdecker (B. 6, 993) found that 67.5 g. S2Cl2, kept at –20° to −22°, absorbed 106 g. Cl in 10 hours, that absorption of Cl then ceased,
and that the liquid had the composition SCl4,. Properties.—A mobile, yellowish-brown liquid; when removed from the freezing mixture used in the
preparation, the liquid gives off Cl, and boils with an absorption of much heat."
Guess on the reaction equation:
SO2 + SCl4 =?= SOCl2 + Cl2O
[Edited on 4-12-2017 by AJKOER]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  |
Per Watts, a curious lesser known path to SOCl2 claimed to proceed by the action of SO2 on SCl4, the latter only existing at low temperatures...
...Guess on the reaction equation:
SO2 + SCl4 =?= SOCl2 + Cl2O
[Edited on 4-12-2017 by AJKOER] |
Thats not a balanced equation. I suspect in reality it is
SO2 + SCl4 => 2SOCl2
Which is obviously more favourable...
[Edited on 4-12-2017 by DJF90]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
DJF90:
If we believe Wikipedia on SCl4 (link: https://en.wikipedia.org/wiki/Sulfur_tetrachloride ), once created we could be done as, to quote:
"It hydrolyzes readily:
SCl4 + H2O -> SOCl2 + 2HCl"
However, too much water and no product:
SCl4 + 2 H2O -> SO2 + 4HCl
-----------------------------------------
Otherwise, I have found one reference citing the reverse of the reaction in question with an equal sign:
2 SOCl2 = SO2 + SCl4
Source: https://books.google.com/books?id=RXFZAAAAYAAJ&pg=PA663&...
So a reaction chamber with pressurized atmosphere of SO2 could move the equilibrium to the left.
Interestingly, a reaction appears to proceed with SO3 also:
SCl4 + SO3 = SOCl2 + SO2 + Cl2
Source: see https://books.google.com/books?id=_TxQAAAAYAAJ&pg=PA452&...)
As such, I feel more strongly with your proposed reaction that creates two moles of thionyl chloride:
SO2 + SCl4 --> 2 SOCl2 (at low temperature)
Note the precise citation by Watts, to quote:
"By the interaction of S02, and SCl4, (Michaelis a. Schifferdecker, B. 5, 924; 6,993)"
There is further support, in my opinion, for the claimed reaction based on the corresponding reaction with phosphorous, namely:
PCl5 + SO2 -> SOCl2 + POCl3
Replacing P with S and substituting 4 for 5 and 2 for the last 3, produces the claimed reaction also.
Interestingly, this possible path to thionyl chloride requires only a drying agent, sulfur, air and chlorine as:
S + O2 = SO2
2 S + Cl2 = S2Cl2
3 Cl2 + S2Cl2 = 2 SCl4 (at low temperatures)
However, -22 F implies the need for some powerful cooling to -30 C. Also, the last reaction may take some 10 hours to perform.
[Edited on 5-12-2017 by AJKOER]
|
|
|