Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Carbon tetrachloride
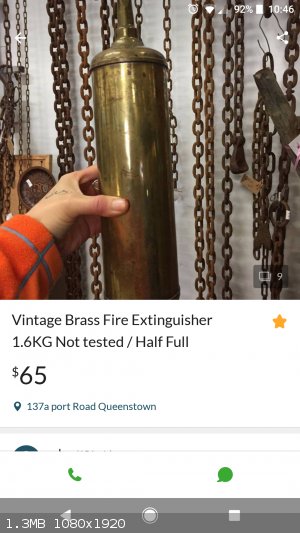
I found on Gumtree this add for an old extinguisher, the type that was filled with carbon tetrachloride. I contacted the seller asking if it's filled
with the original liquid to which he responded 'yes'.
Now to say Carbon tet is rare is an understatement. Australia has banned all imports, exports and uses of the solvent without a license. And no doubt
an expensive license, it's easier to get a license for explosives or radioactive work than it is to work against the Ozone depleting substances Act.
Even my uni won't have any. Shipping of the product is also insanely expensive too, so the value of the carbon Tet is very high.
However, given these laws, that have huge fines if it is used in any way that releases it to the environment, is it worth the hassle? Owning the
extinguisher is probably a fineable offense too, just like owning a Halon extinguisher is too.
I think maybe I might get it, secretly ampoule some for a rainy day, and then make a video out the disposal of the rest? Would that be something that
people would be interested in watching? I still have Halon to dispose of so I'll ask the fire fighter depot what I should do with carbon Tet, no doubt
they'll freak out when they look it up and have absolutely no idea what to do.
|
|
|
j_sum1
Administrator
       
Posts: 6220
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Get it. Ampoule it up. Then sell the empty extinguisher as an antique on gumtree for $65.
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
Due to it's environmental concerns, (Which is fair) I can't expect the availability of it to go up any time soon. Looks big too - I'd ampoule all of
it, possibly in separate smaller ampoules, and keep it in a well packaged, well marked shoe box in the back of a cupboard. You could make some money
selling it in the future, or perhaps find a use for it the doesn't deplete the ozone layer.
It's generally better to have something than to not have it.
Edit:
Oh and i'd watch a video on it, although i'd watch any video of yours.
[Edited on 15-12-2017 by Vosoryx]
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Yeah I mean I would have been very surprised if any Sciencemad people report an answer to the ones above. We all think the same way when it comes to
rare and exotic reagent acquisition (we really are different than the general public aren't we...)
I'll drive down tomorrow. 1 hour drive and then $65 for the cylinder... I hope its not just full of water 
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by Tdep  | Yeah I mean I would have been very surprised if any Sciencemad people report an answer to the ones above. We all think the same way when it comes to
rare and exotic reagent acquisition (we really are different than the general public aren't we...)
I'll drive down tomorrow. 1 hour drive and then $65 for the cylinder... I hope its not just full of water  |
"Original Liquid" - Open to interpretation.
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
You're not wrong, and its a bit of a pricey risk for me to take as I have wasted all my money on other chemicals already. Hopefully I should be able
to feel it tomorrow, it should be noticeably heavier if its carbon tet given its got a density of 1.6
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
Don't do it. You will make a hole in the ozone layer after opening it. .... Just kidding
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
Quote: Originally posted by Tdep  |
You're not wrong, and its a bit of a pricey risk for me to take as I have wasted all my money on other chemicals already. Hopefully I should be able
to feel it tomorrow, it should be noticeably heavier if its carbon tet given its got a density of 1.6 |
Did you ask how full it was? That would also help.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Just got an old carbon tet extinguisher delivered myself.
It's a mid- 20th century version that's canned under pressure and for a single use. (about 500ml)
This means when I open it I can't close it again so I'll be putting a little thought into how to do this without losing too much of it. Maybe
squirting it down an ice cooled condenser into an ice cooled RBF?
Also must check on if a little alcohol will help preserve it like it does with DCM and chloroform.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The vapor pressure of CCl4 at room temperature is 99mmHg. That for water is 24mmHg, and that for ether is 440 mmHg.
So, I wouldn't worry too much about opening that can. I transfer CCl4 at room temperature with no cooling.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Magpie  | The vapor pressure of CCl4 at room temperature is 99mmHg. That for water is 24mmHg, and that for ether is 440 mmHg.
So, I wouldn't worry too much about opening that can. I transfer CCl4 at room temperature with no cooling. |
Thanks. I'm just being overcautious I suppose.
Funny how your perspective on risk changes with time. As a kid I remember most every house had a bottle of CCL4, and people would use it to take spots
out of their clothes; while they were still wearing them sometimes.
Nowadays it's treated like nuclear waste.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  |
Funny how your perspective on risk changes with time. As a kid I remember most every house had a bottle of CCL4, and people would use it to take spots
out of their clothes; while they were still wearing them sometimes.
Nowadays it's treated like nuclear waste.
|
I remember that too! I still go around asking if anyone still has any in the laundry room.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Oh yes, as a kid I played with CCl4 like I play with water. I bought the stuff OTC, in bottles of 100 ml or 250 ml and used it for degreasing things,
washing oil from my hands when they were dirty after repairing my bike's chain, and so on. This was in the 1980's, not that long ago!
At the same time we also bought CHCl=CCl2 and CCl2=CCl2 as OTC degreaser in liter quantities (jerrycans full of them). In a club's cafetaria, big
frying pans (for chips and snacks) were cleaned occasionally with CHCl=CCl2. This works like a charm when resin-like grease/fat sticks to the frying
pan, just a few wipes and it is gone. After cleaning we briefly switched it on to make it warm, not hot, so that the CHCl=CCl2 quickly evaporated.
After that we put in fresh lumps of frying fat (at that time we did not use liquid oil, but big lumps of saturated frying fat). I am quite sure that
due to haste, small residues of CHCl=CCl2 remained before new frying fat was put in the pan.
It is good that these practices are over nowadays, but things now have gone the other way. These compounds are treated like instant death in a bottle.
For some uses they still are useful and as a chemical they are interesting for some experiments.
|
|
|