Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
Does this look Unstable?
I designed this protein this morning and I was wondering if this would be volatile?

It has one Nitrate Group.
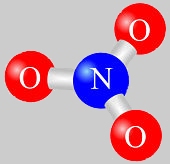
Two Nitrogen Dioxide Groups.

Two Sulfur Oxide Groups.

5 More Oxygen Atoms bound to multiple carbons.
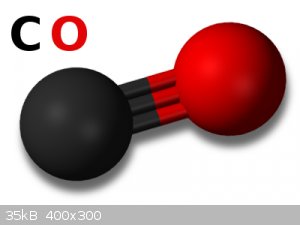
one CO Group and Two CO2 Groups

Then 87 Carbons like a fatty Acid.
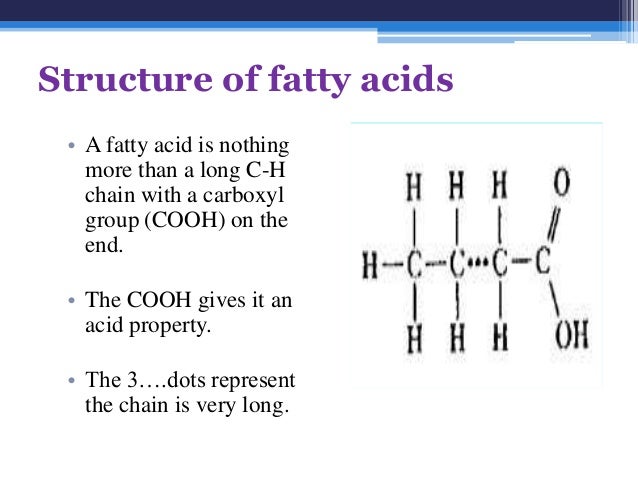
The Ribosome will construct it in a Alpha Helix, but I am uncertain to whether it will explode or actually construct this structure.
[Edited on 15-12-2017 by Vmedvil]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
This is bio chem?? Not pyro.
As to the question...
No idea, but what software you using?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Volatile means that it tends to evaporate, not detonate. How are the nitrogen and sulphur oxides bonded to the protein?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
What's up with the "beta helix" in your diagram? That's definitely not a beta helix you've got there. See:
https://en.wikipedia.org/wiki/Beta_helix
Edit: now that I look at it more closely, it might be a fragment of a beta helix that's shown in your diagram. Is that what it is?
[Edited on 12-15-2017 by Metacelsus]
|
|
|
Texium
|
Thread Moved
15-12-2017 at 09:21 |
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
To Answer both your questions,This is a Synthetic Protein, They are bonded to carbon, so covalent. Sorry, That was a Typo, it is a Alpha Helix not
Beta Helix.

And to answer Nemo's Question YASARA
Yasara Website
[Edited on 16-12-2017 by Vmedvil]
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
I am just wondering if it will explode or is an organic Explosive, it has Two Nitrite groups and one Nitrate group on each repeat of 10 amino acids.
Nitroglycerin has 3 Nitrate groups per repeat.
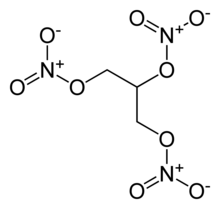
RDX the explosive part of C-4 has 3 Nitrite Groups
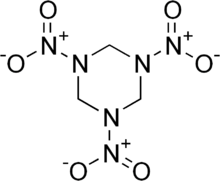
Along with Two sulfur Monoxides which is highly flammable turning into Disulfur Dioxide when burned.

It also has two CO2 and 1 CO group, will this go through oxidation/Reduction, Which Carbon Monoxide is highly flammable.

All of which are highly Toxic too as gases.
In Any case, This is the E.coli Only gene for it, it will make 90, 12 unit proteins per visit by RNA polymerase or 1080 units in 90 separate helix.
E coli Promoter Region (trp)
1 caaatattct gaaatgagct gttgacaatt aatcatcgaa ctagttaact agtacgcaag
61 ttcacgtaaa aagggtatcg acaatgaaag caattttcgt actgaaaggt tggtggcgca
121 cttcctgaaa cgggcagtgt attcaccatg cgtaaagcaa tcagataccc agcccgccta
181 atga
Helix 2 Turns
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
tattgcatgtttcatgtgaactgctattattattgcatgtttcatgtgaactgctattat
Terminator Region
1 cctgatagg
as I said the Ribosome will try to construct it.
I also made a 200 turns Version with the same Promoter and Terminator 1200 units 90 times, so 108,000 Units per RNA Polymerase in 90 separate proteins
of 200 Turns which would not fit in this post because it is 29,353 Base Pairs versus 554 Base pairs.
[Edited on 16-12-2017 by Vmedvil]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Two nitro groups and a nitrate per ten amino acids isn't going to make it explosive.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
Well, I don't know it also has 87 carbons so maybe not. C87,N3,S2,O14
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Are you trolling? If you don't have more oxygens than carbon, it's not going to be an explosive.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
To be fair, they are nice images.
Google Image Search is really quite good these days. Just by pasting in the image location (right click over the image and select 'copy image
location') the source(s) of the image(s) is/are quickly identified.
Unlikely Vmedvil is doing anything at all, seeing as the first hit on the big image is "Biology4Kids.com: Cell Structure: Ribosomes"
[Edited on 16-12-2017 by aga]
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
Well, the other ribosome image would not work that I wanted to post. Kids learn the same thing just not as detailed.
This is the one from Biology Dictionary.
[Edited on 16-12-2017 by Vmedvil]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
How about designing an enzyme that makes acetone peroxide? Acetone can be biosynthesized from acetoacetate decarboxylation, and hydrogen peroxide can
be made by partial reduction of oxygen, as occurs with many enzymes (for example, pyruvate decarboxylase).
Now, just put them together, and you have your biological explosive. (Don't try this at home, kids.)
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
That would be too easy if there are already proteins that do it, all you would have to do is put the two active sites right next to each other.

[Edited on 17-12-2017 by Vmedvil]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Actually Doing It would be less easy right ?
Another cut-n-paste artist.
Great images copied from other websites, but no Action. Ah well. Same old same old.
One day soon an SM member will Do something truly amazing, i feel sure.
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
What makes you think I would share that with you even if I did do it?
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Question
How would you go about making yeast fart methane instead of CO2?
|
|
|
Vmedvil
Banned for blowing margins instead of minds...
Posts: 42
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
That would require a more extensive rewrite then one protein like the Bio lumen Energy Pathway.
Bio Lumen Pathway
[Edited on 18-12-2017 by Vmedvil]
|
|
|