| Pages:
1
2 |
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Inert test tubes & teflon (or glass) cap liners.
I have been hunting on ebay for small cheap vials in the 8 to 16mL range to use in lieu of traditional test tubes with rubber bungs. The rubber bungs
pop out under low pressure and aren't chemically inert, anyway. I've found vial with phenolic caps that it can take heat up to 220C before breaking
down. This allows me to melt urea and other substances in a tube that is sealed off from air and do non-aqeuous chemistry. However, I haven't been
able to find low cost phenolic capped vials with teflon sealing liners (or teflon plugs like a stopper in addition to a screw on cap). Phenolic resin
isn't chemically inert. The manufacturer of the vials was also inconsistent, some of them have a plastic foam liner ... some of them don't. (see
picture). But it's not PTFE... so I might as well assume it's not chemically inert and remove the liners anyway.
I searched sciencemadness and ebay for suggestions on how to get low cost teflon cap liners that would seal a dram vial, but it's a no go. I found
that lots of users had problems with using teflon tape or commercial caps with pre-made liners since the teflon was not always pore-free. (porous
teflon leaks gasses.)
White teflon tape for plumbing from the hardware store is porous, and several people noted that caustic chemicals or gasses can get through the tape
if it's used as a liner. ( Goretex is a form of PTFE too ... it passes gas, but not liquids.)
I've seen several thicker teflon tapes, such as a natural gas sealant tape which is supposed to be impermeable. However, these are colored yellow and
I have no idea if the coloring is chemically inert.
I've seen some tapered ground joint liners for $40 / set of three, but that kind of price is riduculous for a piece of teflon paper.
This seems like such a common problem that there has to be someone who sells just plain teflon cap liners; or someone can tell me what colors of extra
thick teflon tape are the most chemically inert ... or explain a way to get a cheap punch and a thick white teflon sheet for a reasonable price.
Does anyone have good pointers on where to buy/make/find a low cost circular disk liner or plug that can handle heat to 220*C, and caustic chemicals?
I'm looking for solutions under $20 for 25 vials.
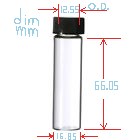
I bought 24 vials for experimenting from ebay seller homeluxe-grandparfums . I'm not entirely sure vial dimensions/threads are standard and the glass
is quality. I just bought them and measured them with a precision dial caliper. The cap insides have about a 13.45mm (~0.525 U.S.A. inch) diameter to
the threads; so that's the max size circular disc that will fit. The vial's glass neck (minimum O.D. not including threads) is 12.55mm. That's the
minimum size disk I would want so that it compresses properly. (1/2 inch.). The inner neck diameter for a plug is 9.40mm ( 0.370 inch).
The vials are only worth half a dollar each. I want low cost vials, so that when I do experiments that are really hard to clean up ... I can just
throw away a vial and not feel bad about it. But if there are teflon sealers for a different vial at low cost, I would consider switching vials
(especially if they are borosilicate).
The only other thing I can imagine might work, is if there was someone who sold borosilicate glass disks that were thick enough to take the stress of
being compressed in a cap without cracking. In that case, I could put a little silicone in the phenolic cap to glue them in, and put a little glass
etchant on the lip of the vial. Then after screwing the cap on, the etchant it would naturally frost only the sealing contact points making it like
ground glass.
Anyone have other ideas?
[Edited on 17-12-2017 by semiconductive]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
That's a whole lot better than the glass shattering.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
I have Erlenmeyer flasks with keck-clips to hold ground glass. Even at full boil of water, they don't shatter. There's pressure release. So, not
all glass shatters at high temperature or even pressure if set up correctly. Urea does not generate much pressure, and never shatters glass: note:
That's one of the chemicals I mentioned in the O.P.
However: There are techniques I have developed to handle vials as well under pressure even with cheap and thin walls.
Vials can be put in a sealed thermal chamber with silicone oil over the top of them and no air space. Since silicone oil is incompressible, the vials
are not able to shatter ... they are only able to be contaminated by silicone oil if there is any leakage. And if the chemicals are corrosive or
damaging to phenolic caps ... it's a matter of time before they weaken the cap due to chemical attack.
Borosilicate glass is far better than normal glass when it comes to handling shock resistance and leaching of alkali into solutions. That's the only
reason I mentioned that I would rather have definite borosilicate than regular glass.
From the data I have seen on glass corosion, there are other glasses that are even more resistant to alkali attack than borosilicate. Kimble glass
)Tm) , for example, etches much slower than than Pyrex (Tm) according to data easily found on the web.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
The vials sold to me show between del = 0.0005 and 0.0008 inch expansion, on 3.7445 inch length from 21.5*C to 80*C.
del / 3.7445 / (80-21.5) = 2.28 to 3.65 ppm.
Soda lime glass has 8.5-9.5 ppm expansion and borosilicate has 3.3 ppm.
So, I believe that ebay seller: homeluxe-grandparfums is presently (Dec 2017) selling cheap borosilicate vials.  YAY! YAY!
I am looking for punches to make 0.5 inch AKA 12.7 mm cuts from teflon tape. If no one has any leads on pre-made teflon liners, I'll make my own and
test them for chemical resistance.
I was thinking a drop or two of hydrogen peroxide (3%) mixed in with fuming sulfuric acid might be a good test of oxidizibility. Can anyone think of
a more corrosive substance that can be made from hardware store chemicals to test the quality of teflon that various tapes are made of?
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Qorpak sells PTFE liners.
Oh and I'd use something more concentrated than 3% hydrogen peroxide... I typically use 3 parts by volume concentrated sulfuric acid (fuming sulfuric
acid is slightly stronger but not by much) and 1 part 30% hydrogen peroxide.
[Edited on 25-12-2017 by JJay]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by JJay  | Qorpak sells PTFE liners.
Oh and I'd use something more concentrated than 3% hydrogen peroxide... I typically use 3 parts by volume concentrated sulfuric acid (fuming sulfuric
acid is slightly stronger but not by much) and 1 part 30% hydrogen peroxide.
[Edited on 25-12-2017 by JJay] |
Thanks! I found quorpack, and they do indeed sell green thermoset caps with PTFE liners for a quarter dollar each. I'm not sure what threads the
vials I have are .. but even if the caps don't fit the vials I got, the liners will work ... so it's worth buying a set to test.
I don't see H2O2 in concentrated form, locally. I'd have to evaporate some 3% down or mail order it. Mail carriers seem to get antsy over 30%
peroxides and charge more, so I'll test a few drops of the 3% stuff first to see if there's any obvious effect.
I put the yellow teflon "full density" gas tape into 97% sulfuric acid (Rooto from ACE hardware), and am letting it set to see how much dissolves.
Before I put the tape in, I could see it was white near the edges and yellow in the middle; so I think the yellow color may be mostly a surface dye.
Once in the acid, the tape immediately turned from yellow to dark orange in some places near the middle of the tape. I think something is also
diffusing into the acid by the look of it ... If the color is just a surface coating, maybe most of it will dissolve off.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
If your Rooto is 97%, you got a good batch. I find that it is typically around 90% when analyzed using the best methods I have available around the
lab. It contains inhibitors.
I think you can ship small quantities of hydrogen peroxide ORM-D. I get mine from a hydroponics store.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
I just looked the MSDS up, and ROOTO is supposed to be 92.3%. So your typical analysis is closer to what it's supposed to be.
The MSDS doesn't mention any inhibitors; do you have any idea what kind of chemicals are used for inhibitors and why? ( I plan on using ROOTO to
clean vials after organic chem, to get rid of trace amount of oils. )
Note: The yellow gas tape turned a fairly uniform brown after 24 hours. No further leaching occurred after the first half hour or submerging in
sulfuric acid.
I added 1 drop of H2O2, 3%, which of course got very warm ! 
It didn't fizz, though. The tape started to turn back to yellow toward the top of the vial where the drop of H2O2 was added.
Then I added 1 drop of ~43% HCl. The tape turned more yellow at the top.
Then I added a few crystals of KNO3, hoping to relase nitrate ions and make a weak aquia regia type mixture. It outgassed very rapidly, and there's a
good chance that all the NO3 was converted to oxygen + nitorgen by heat. I don't know. But, there was no noticable change on the tape. It's still
yellow near the top of the vial, and uniformly brown all the way to the bottom. Even after 12 hours, the situation has not changed. ( Edit: I spoke
too soon, the tape is suddenly starting to turn white, 20 hours after adding the K2NO3. )
So, the yellow dye can be leached. eg: It can probably be "pickeled" to be less chemically active. ( I plan on trying sodium Hydroxide later, as
well. )
Regardless of the outcome, using tape to make liners isn't a very practical idea. I bought a paper punch with 1/2 inch diameter. It very crisply
cuts paper discs. However PTFE tapes (The normal white kind) are extremely thin, and tend to tear into strings that align with lengthwise with the
tape. OTOH; The yellow gas tape is thicker, and does punch cleanly most of the way around. But since the tape itself is only 1/2 inch wide, it's
impossible to align it and a very ragged cut always happens near the sides of the tape which gets pulled into the cutter.
So, basically, to be able to punch PTFE would require full density teflon sheeting that is on the thicker side (say 0.003 of an inch thick, ~0.08mm )
and significantly over half inch wide (>> 12.7mm) in order to punch cleanly. That's not available locally in my hardware stores, so it's an
ebay or mail order item; buying qorpak caps is likely the best solution.
Doh!!! Qorpack caps also use the yellow colored PTFE. !!! , So, I'd still have to pickle them before using them on strong acids. 
Thanks for the help.
[Edited on 30-12-2017 by semiconductive]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I saw the name of the inhibitor stated at one point but the source was a bit dubious, and I have not been able to find it again... but I have seen for
myself that Rooto definitely contains unwanted impurities: https://www.sciencemadness.org/whisper/viewthread.php?tid=75...
If you want really clean sulfuric acid and all you have is drain cleaner, you will have to distill it. Everyone wants to avoid doing that, and there
are 20 different YouTube videos claiming that the channel owners can obtain pure sulfuric acid OTC by some cheap and easy method that doesn't involve
distillation, but they are all wrong.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
I bought rooto because several people on Sciencemadness mentioned it as good quality.
Mine happens to be a very clear solution, which really looks like distilled 98% solution. But you're right, it likely needs distilation to be used
analytically. I do have a 300mm Vigerux column, and 300mm Allihn condenser, but the distillation temperature is daunting ... 218 to 260 Celsius. (
500 Farenheit.) I've only got an alinco V stir rod ... and I think it might demagnetize at a temperature somewhere near that point.
I'm wondering about your thread; wouldn't 90%+ of H2SO4, distilling to an acid with only 8% or so more density?
What do you mean in that thread by "distilled to 1/3rd it's volume?" do you simply mean that ~2/3rds of the solution is distilled into a separate
container, while ~1/3 remains in the boiler flask? eg: is the sludge in the boiler?
The color change of the precipitate reminds me of zinc oxide; ZnO turns yellow as it gets hot but is white when allowed to cool off. At the
temperatures required to distill sulfuric, I imagine a yellow tinge would be noticable on zinc oxide and probably zinc sulfides.
I'm not sure if the color I saw on the tape could be related to impurities or not.
The yellow Teflon tape I tested turned dark streaky brown when hit with Rooto, and then after other acids are added the brown leaves the tape and
drifts into solution (see picture). Most of the brown leachate precipitates to the bottom of the vial, but enough is still in solution to make the
rooto turn slightly brown. But the tape is nearly white compared to the yellow it started out with.

This makes me wonder what the dye could be. I tend to think that organics would generally be oxidized by high concentration sulfuric acid with
hydrogen peroxide added. I would expect them to turn to carbon, or hydrocarbon gasses. When you say you've seen techniques to purify sulfuric on
youtube, did they try adding hydrogen peroxide before and after condensing the sulfuric to precipitate and destroy orgnaics?
I'm mostly interested in strong sulfuric for cleaning purposes; like getting rid of traces of oil on glassware, etc.
Although I could distill the Rooto, in the end, I would still also have to put it into another container ... because who knows if the impurities are
actually part of the bottle or filler in the plastic Rooto uses. I'm not good enough with chemistry to be able to test it yet.
I have NAPA auto store battery acid ( 25 - 30% H2SO4) in another container. I'm guessing that would have to be quite pure because car batteries are
ruined by side reactions caused by impurity ions like sodium, etc. The NAPA auto battery acid bag has held sulfuric for a long time but with a lot of
water in it. I'm not sure how much the properties of H2SO4 change when it hits that 98% borderline fuming range. I've had the bag for over seven
years ... which shows how slowly I've been using it; but I'd hate to distill the stuff just to have it ruin the bag; Watering it back down after
going through distilling it seems like a waste too.
[Edited on 3-1-2018 by semiconductive]
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by semiconductive  | | I've seen several thicker teflon tapes, I've seen some tapered ground joint liners for $40 / set of three, but that kind of price is riduculous for a
piece of teflon paper. |
Those tapered ground glass joint liners: I bought a set of 10 for about $35, years ago, and I virtually never use them. They don't seal particularly
well, and they tend to be arduous to remove afterward (crinkling up, etc.). I don't recommend them unless you have an absolute need to avoid grease in
your setup--and even then, it'd be worth the extra expense to buy the super pricey fluorinated grease just to avoid the hassle that is working with
them.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
semiconductive, you seem to be doing pretty good at chemistry so far.
B.P. of sulfuric acid is a good bit higher than you specify, though. Sorry to burst a bursted bubble further.
When a binary solution is boiled, the more volatile component leaves at a higher rate. The lower boiling compound usually leaves a bit, too. The
distillate will have more of the more volatile compound, and the boiling flask or "bottoms" will have more of the less volatile component. "Distilled
to 1/3 volume" probably means that it may or will take distilling off 2/3 of your total liquid before your bottoms will reach a concentration of 98%
H2SO4, the azeoptrope (I dunno because I didn't check the thread). You can also concentrate it on a beaker on a hotplate, outdoors or in a hood,
although I have never done it I am sure it is pretty straightforward: drive off water until you reach a room temperature specific gravity of 1.84, I
think.
Edit: Though I have never done the beaker method, I would probably use heavy boiling chips (porous ceramic chunks, broken coffee mug) because H2SO4
bumps like something I've never seen. Regular boiling chips float on the surface of the acid.
[Edited on 1-3-2018 by happyfooddance]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I mean when 2/3 of the volume is distilled off, a precipitate forms. It is likely largely ammonium bisulfate from the destruction of the inhibitor. It
is widely believed among hobbyists that Rooto is high quality sulfuric acid, but this is a myth.
The specific gravity of high strength sulfuric acid is not really a good indicator of its concentration either; if you want to verify that it is 98%,
you will have to do a titration. Check some density tables and check the precision of your equipment, and you will see what I mean.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by weilawei  | Quote: Originally posted by semiconductive  | | I've seen several thicker teflon tapes, I've seen some tapered ground joint liners for $40 / set of three, but that kind of price is riduculous for a
piece of teflon paper. |
Those tapered ground glass joint liners: I bought a set of 10 for about $35, years ago, and I virtually never use them. They don't seal particularly
well, and they tend to be arduous to remove afterward (crinkling up, etc.). I don't recommend them unless you have an absolute need to avoid grease in
your setup--and even then, it'd be worth the extra expense to buy the super pricey fluorinated grease just to avoid the hassle that is working with
them. |
That doesn't surprise me. I am hoping to figure out a way to slightly regrind glass joints using fine silicon carbide grit to improve glass joint
seals, and after connecting the joints -- wrapping the outside of the pipe with teflon tape a lot of times (I've got two rolls of Teflon with nothing
to do...and it's wasted money anyhow.);
I don't have the hands on experience with all the lab equipment yet; but for most of the stuff I am planning to do, I think plain silicone oil may
work OK as a grease; as long as I can clean it off afterward.
For the little glass vials, I just ordered two virgin white PTFE sheets at 0.3x100x100mm and 0.5x150x150mm respectively from China. Since I only need
to make half inch disks to seal the vials, even 100mmx100mm sheets should easily make 24 to 36 cap liners by punching. (I only need 24 total. )
The thicker PTFE sheet is just in case I don't get perfect punches from the thinner sheet. I'll let everyone know which works best ... and I suppose
I could sell off any good extras to recoup some of my costs for the punch and wasted tape. (P.M. me as customelectronic on ebay if you care). I
think vials have pretty standard neck sizes, so 1/2 inch (12.7mm) necks should be fairly common.
I also have a way to make a silicone backing to imitate the foam backing that my vials were sold with, but with better chemical resistance. With that
for a spring loading and a Teflon liner, I don't think I should have any serious risk of chemical contamination problems or cap failures.
Even if the PTFE material isn't "full density" its extremely thick compared to teflon tape and no porosity issues or chemicals from seeping into the
phenolic caps. I'll let everyone know how it comes out after I test them.
[Edited on 3-1-2018 by semiconductive]
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JJay  | I mean when 2/3 of the volume is distilled off, a precipitate forms. It is likely largely ammonium bisulfate from the destruction of the inhibitor. It
is widely believed among hobbyists that Rooto is high quality sulfuric acid, but this is a myth.
The specific gravity of high strength sulfuric acid is not really a good indicator of its concentration either; if you want to verify that it is 98%,
you will have to do a titration. Check some density tables and check the precision of your equipment, and you will see what I mean.
|
I see. Again, sorry, I didn't check the thread he was talking about, I made an assumption about what I thought he was talking about.
You are correct, I always rely on a titration, even when a thermometer says 335 for 12 hours. Carefully neutralizing and weighing the residue works
well, I usually do these and a few other analytical things, just so I can sleep well at night. I mean, spending all day and risking life and limb to
distill this stuff, it isn't really going much farther to check its purity.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by JJay  |
The specific gravity of high strength sulfuric acid is not really a good indicator of its concentration either; if you want to verify that it is 98%,
you will have to do a titration. Check some density tables and check the precision of your equipment, and you will see what I mean.
|
Right now, I'm having trouble being sure of the density measurements for plain water. See the end of my other thread on volumetric flasks.
https://www.sciencemadness.org/whisper/viewthread.php?tid=78...
I've figured out that air dissolution really messes up density measurements of most liquids.
I'm not finished with that thread, so answering your post is a bit of a chicken and egg problem as there will be more posts. I've been testing
Henry's law at boiling, and comparing data sets with those made by other people like engineering toolbox, but I managed to delete my gnuplot graphs so
it's going to be a few days before I can get it all back and re-enter data.
The problem is that gas dissolution charts expect "equilibrium" conditions, and steam flowing away from a flask is not equilibrium ... So the meaning
of the charts showing de-gassification by boiling is very tricky to compute; it requires the partial pressure of steam to be corrected for how much
gas is actually trapped over the liquid at equilibrium.
On the other hand, liquid is *SURPRISINGLY* susceptible to volumetric distortion by dissolved gas, and potentially allows very sensitive and cheap
tests to be developed based on volume rather than full titration. But, I'm not there yet. Even water is very non-linear in it's expansion with
temperature, and reviewing thermodynamics after 25 years is a bit slow for me. I'm used to doing statistical mechanics at the quantum level, and the
crude thermodynamics used for water and chemicals is just painful.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
OK. The virgin teflon sheets came in the mail, finally .... only about a month later.
The sheets were obviously cut from a roll, because even though it was put into a cardboard press in the mail to keep it flat ... it still curled when
removed. The mail package wasn't damaged, but there are also signs of dents on the surface. Ironing uncurled the teflon, but did not remove the
shallow crease marks. SInce none of marks are deep, they don't appear to affect sealing of the flasks any. The glass vial lips will crush the teflon
and replace the crease marks with lip dents ... so I don't think the wrinkles matter.
It turns out that either thicknesses of virgin teflon punches perfectly. I have extra teflon left-over even after making 24 liners for my bottles.
If anyone wants them ... contact me privately. I'm happy to punch and get rid of the rest of the teflon for a worthy amateur just to recover my
costs. I will do transactions on e-bay, so you are protected. It's going to be about 10 cents each (US) for the 0.3mm thick punched liners, and 25
cents each for the 0.5mm thick punched liners. They are presently punched at 1/2 inch (12.7mm) diameter which is typical for vial necks. If you
want a different diameter, it will cost more because I'd have to get a different punch.
I can also coat one side of them with silicone to act as as a rubber spring to aid in sealing if anyone cares for the extra; just add $1.00 to cover
the silicone cost.
  
[Edited on 23-1-2018 by semiconductive]
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by JJay  | I mean when 2/3 of the volume is distilled off, a precipitate forms. It is likely largely ammonium bisulfate from the destruction of the inhibitor. It
is widely believed among hobbyists that Rooto is high quality sulfuric acid, but this is a myth.
The specific gravity of high strength sulfuric acid is not really a good indicator of its concentration either; if you want to verify that it is 98%,
you will have to do a titration. Check some density tables and check the precision of your equipment, and you will see what I mean.
|
I've been trying to distill rooto at 150Celsius after adding hydrogen peroxide, and then later I tried adding some distilled water; I just wanted to
to see if a bad distillation setup would work at lower temperature with moisture added. It does work (barely), and if I use a fish tank pump to move
air across the surface of the hot sulfuric acid ... much more of the acid gets carried up the still and condenses in a standard allihn condensor. The
water/peroxide doesn't even appear to be critical.
Unfortunately, without a piece of glass to force the acid to go through a "wet" spot during condensation, about half the acid stays in the air and
gets out of the still and into the room where the still is causing a choking gas to appear. When I put a silicon carbide ball bearing on the vacuum
take-off, almost all of the acid is forced to go through the acid pool where the bearing touches the cold glass. That seems to be enough to get
almost all the acid out of the air. It can still be smelled ... faintly ... but it's at a very low level and not choking... the air exchanger is only
six feet away for the whole house and it seems to be doing its job. No smell when more than 10 feet away from the still, even after running for a
week.
I learned the hard way that concentrated sulfuric acid attacks silicon carbide and some of it dissolves into the collection jar. Once the surface of
the marble became clear with silica only ... the dissolution appears to have drastically slowed down or stopped. The concentrated sulfuric in the
condenser is perfectly clear ... but there is a slight brown from the silicon carbide in the collection jar.
I refluxed old 3% hydrogen peroxide solution at 120Celsius for a day to concentrate it before adding any to the acid. H2O2 bottles were over a year
old, but about two quarts of solution concentrated down to just under 200ml after cooking for a day at 140Celsius with a vigereux column above it. I
figure its > 15% now because even wiping off the surface moisture from a glass rod turned my skin instantly white.
After letting the H2SO4 cook with H2O2 (just a few drops) for a day or two I could see small impurities floating around in the solution, crystallized.
So, I'm backing you up on your find that there is something in Rooto in spite of it being very clear. It's not pure sulfuric, whatever they added. I
can't get a picture of the floaters as they don't show up in the camera easily.
I'm thinking of making a water-glass frit and silicone exit plug to allow air out ... but preventing strongly polar chemicals from getting out in the
air. (Silicone has non-polar bonds, so strongly polar acid gasses like sulfuric ought not want to go through.)
Lower temperature distillation seems possible, if the still is properly constructed and air is used analogous to steam distillation.
Unfortunately, the European 24/40 joints I have are not truly ground but stamped on a machine. I can see air bubbles in the joint where there are
small gaps in the tapered surface. A tiny bit of sulfuric can work its way out the joints through those concave areas .. say a drop every four hours
or so. It ruined the washcloth that I was using as an insulator to keep the boiling flask temperature up. Sigh.
I've got some valve grinding silicon carbide for a car engine from NAPA ... perhaps after crushing it fine with a hammer I can use that to regrind the
glass joints against each other to get rid of the slight concave points that are leaking acid. The only other thing I can do is use my welding torch
to heat the inner tube of the allihn condenser, and widen the tube at the mouth so liquid isn't trapped there against the glass joint in the first
place. It seems that allihn condensers aren't very carefully designed to avoid trapping fluid....
  
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by semiconductive  |
I've got some valve grinding silicon carbide for a car engine from NAPA ... perhaps after crushing it fine with a hammer I can use that to regrind the
glass joints against each other to get rid of the slight concave points that are leaking acid. The only other thing I can do is use my welding torch
to heat the inner tube of the allihn condenser, and widen the tube at the mouth so liquid isn't trapped there against the glass joint in the first
place. It seems that allihn condensers aren't very carefully designed to avoid trapping fluid....
|
I wouldn't bother grinding those joints any more; they are only airtight if they are sealed with grease or something. Pretty much the only oil or
grease that would seal effectively at those temperatures is sulfuric acid, and even it would be boiled off pretty quickly.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Teflon tape will seal the joints without decomposition.
|
|
|
Deathunter88
National Hazard
   
Posts: 508
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
I don't think allihn condensers are used for simple distillations, rather refluxing in the vertical orientation.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
That would explain the problems I'm having. I am a chemistry newbie in 2017. I hope to improve, quickly in the coming months. Thanks for pointing
that out. I was sold one becuase it was supposedly "more efficient" than a straight liebieg and easier to clean than dimroth ... and I thought I was
buying the best general purpose condenser. Now I see there was a mis-commmunication.
I wonder which is better to keep for refluxing ... the vigereux column, or the allihn. I bought the vigereux in the pictures with the idea that it
would increase the number of times the fluid condensed and re-evaporated so that it effectively acted like I was doing multiple distillations of the
same substance. In the picture, I'm just trying to use it to recycle some of the water and increase the SO4 that is dissolved in it by taking the
distillation tap off the bottom of the column.
I am also worried about cleaning the vigereux because it's hard to get a brush onto all surfaces. I bought it because I was told that that the bent
inward glass gave bigger surface area near the outside to condense on and is an insulation feature furthur in which helped aid in re-evaporating the
fluid after being condensed. I was considering adding an insulation jacket to the outside of the vigereux column, and then using a thermostatically
controlled air pump to precision control the outside temperature of the column. (Computer control.)
But it seems like the allihn might be able to do essentially the same thing and be easier to clean. It could be insulated on the outside, and a
temperature probe put on the top and bottom to electronically control the temperature gradient.
Do you have any idea if a Vigereux has any special advantages over a Allihn, when it comes to acting "as if" multiple plates/distillations are taking
place? (I'm wondering what other mistakes in my thinking there may be.)
[Edited on 25-1-2018 by semiconductive]
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Allihn are great when packed. You can do lots of things with them. Usually in reflux orientation...
Every once in a while you will need to resort to chemical cleaning for your more intricate glassware, but a big part of chemistry is knowing how badly
a piece will be soiled by a given operation: and proceeding accordingly. Some reactions would be part of the literature, because they work, but that
the resulting mess renders the synthesis inefficient. Many experiments yield much knowledge because of the time you spend thinking about how you
fucked up while you clean your glassware.
For refluxing, I like the vigreux, because you don't need a water source. For some things. But a leibig or west is better overall.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
A Liebig (or better, a West) is the best general purpose condenser.
Dimroths are hard to clean but are more general than Graham condensers and more efficient than Allihn condensers. I mainly only use my Liebig and my
Dimroth. But I also have a couple of Vigreux columns... they are better than nothing for fractional distillations. Packed columns are very
efficient... probably more efficient than some of the fancy expensive columns.
|
|
|
semiconductive
Hazard to Others
  
Posts: 287
Registered: 12-2-2017
Location: Scappoose Oregon, USA.
Member Is Offline
Mood: Explorative
|
|
Quote: Originally posted by JJay  |
I wouldn't bother grinding those joints any more; they are only airtight if they are sealed with grease or something. Pretty much the only oil or
grease that would seal effectively at those temperatures is sulfuric acid, and even it would be boiled off pretty quickly. |
The joint that's leaking acid actually stopped once I rotated it back and forth a few times while pushing the glass together. In the bottom of the
picture I took, you can see a large air bubble (diameter wise, not thickness wise) under the condenser where the acid is leaking out. That bubble
with its striasions takes up over half to 3/4 the ground joint length ... so the seal is only effectively along 1/4 to 1/2 of the ground joint's
potential contact length.
I've got silicone oil and also PTFE plumbers paste. The oil's no good because liquid is being forced out of the joint; but I think the paste being
teflon particles, would probably seal the joint. It's just a pain to use if I don't have to.
One thing I noticed ... I can put my finger over the vacuum outlet for a few seconds and then release it. When I let go, I can feel a strong puff of
air because the pressure built up from the aquarium pump. So, it does appear to be pretty air tight -- if not a perfect seal.
The salesman did say the bigger joints were supposed to be better at sealing than the standard smaller American joints; so that's why I chose to
standardize my glassware to one size -- 24/40 ground glass; wherever possible. Apparently the salesman was at least partially right?
[Edited on 25-1-2018 by semiconductive]
|
|
|
| Pages:
1
2 |