Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
My Senior Thesis: Removable Linkers for the Hexadehydro-Diels-Alder reaction
I recently presented my Honors thesis, and I thought that Sciencemadness people would also be interested in seeing it, so I am posting it here.
Abstract:
In the hexadehydro-Diels-Alder (HDDA) reaction, a diyne and an alkyne react in a formal [4 + 2] cycloaddition to produce an o-benzyne intermediate,
which can be trapped to produce a wide variety of substituted arenes. For reasons of kinetics, the HDDA reaction is only practical as an
intramolecular reaction with a linker between the two reacting groups. To date, only a small number of viable linker classes are known. Because the
linker remains in the product after the reaction, this limits the types of compounds the HDDA reaction can produce. In the current work, sulfur-based
linkers were developed which can be removed by reductive desulfurization after the HDDA reaction. These linkers were constructed using alkynethiolate
chemistry, enabling them to be directly synthesized from terminal alkynes or diynes in a one-pot reaction. Silicon-linked compounds were also
investigated, but they were found to be unreactive under both thermal and photochemical HDDA conditions. Overall, these results help to delineate
which linkers are feasible for the HDDA reaction, and also provide a new synthetic strategy that broadens the set of compounds accessible via this
reaction.
(Edited at OP's request due to potential copyright issue)
[Edited on 5-2-2018 by Bert]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Very cool and incredibly impressive research for an undergrad. Here I thought you had a PhD already.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Thanks!
I've applied to various chemical biology PhD programs, and I will start as a graduate student next fall.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Very nice, the HDDA reaction has struck me as an interesting reaction. Although I always took that it was for the synthesis of highly functionalized
arenes. But Indeed using a thioether linkage and removing that removes this restraint.
I have been following literature on the HDDA reaction, although not that much recently. Now I remember there being significant radical character to
the transition state(s) and or intermediates of the HDDA. I have read (but need to re-read) this paper (Which you also cite in your thesis: http://pubs.acs.org/doi/abs/10.1021/acs.joc.5b01356).
If you argue that N-Ts and N-Boc are not EDG then this is, to my knowledge the first report of ultilizing electron rich alkynes/butadiynes in the
HDDA.
On paper I've always pondered about the feasibility of the HDDA in the attached picture.
Still haven't figuered out (on paper) which group to use as that X. Several groups can stabilize adjecent radical centers, both EDG and EWG. The
question I wonder about is how much stabilizing would you need for this HDDA and which group would be suffucient? Would there be differences between
the thermal and photochemical reaction?
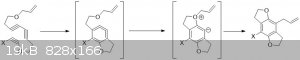
[Edited on 19-12-2017 by Sigmatropic]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
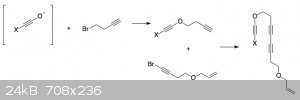
I think what you want to do might be possible, although I have no guarantees.
To make your starting material, the path shown in the image might work.
There's no literature precedent for the type of linker you plan to use. I think it would probably work OK by analogy with nitrogen-based linkers, but
it would likely require high temperatures (~150 °C).
Once the benzyne is formed and initially trapped to the oxonium zwitterion, it could form your desired product by transfer of the allyl cation.
However, the allyl group might also be removed by transfer to solvent (as seen with the methyl oxonium ion in my reactions). You'll want to choose a
solvent that can't easily exchange a proton for a carbocation. I would recommend something like 1,2-dichloroethane.
[Edited on 12-20-2017 by Metacelsus]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I have already spent some time thinking over the synthesis of the starting material, although I will always take a good look at creative approaches. I
will not discuss it here in depth seeing the use of the hypothetical product of this HDDA.
What I will disclose is that it starts (well there's two more steps to start from raw materials) from a symmetric alcohol and one of the last steps is
the introduction of the ynol ether. This last step is quite envolved as there are many acidic protons, atleast to the base employed. As a direct
result of this the usual electrophilic quench with "X+" will probably not work and have to substituted for by a 2-step procedure to introduce X.
You mention the methyloxonium produced in the reaction(s) in your thesis were intercepted by solvent molecules. Did you observe 1,1,1-trichloroethane
or MTBE or were they inferred? Now that I think of it the products are too volatile to be easily detected.
Mechanistically speaking 1,3-alkyl migration involves inversion at carbon (and thus very high activation energies), whereas 1,3-silyl migration (Brook
rearrangement, proceeds through a hypervalent oxasiletane) and 1,3-allyl migration (a variant of the claisen rearrangement, proceeds through a
6-memberred transition state) should have lower transition states and should proceed more readily than solvent interception.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Mind boggling.
As a mere novice, it is awe-inspiring trying to read, and partially understand your thesis.
Having to resort to google to decipher even the title was an indication of the difficulty level.
Thank you for posting it here, and congratulations on what looks like excellent work.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Sigmatropic  |
You mention the methyloxonium produced in the reaction(s) in your thesis were intercepted by solvent molecules. Did you observe 1,1,1-trichloroethane
or MTBE or were they inferred? Now that I think of it the products are too volatile to be easily detected.
Mechanistically speaking 1,3-alkyl migration involves inversion at carbon (and thus very high activation energies), whereas 1,3-silyl migration (Brook
rearrangement, proceeds through a hypervalent oxasiletane) and 1,3-allyl migration (a variant of the claisen rearrangement, proceeds through a
6-memberred transition state) should have lower transition states and should proceed more readily than solvent interception. |
In other work (not with the compounds I described in my thesis), I detected 1,1,1-trichloroethane by NMR after running the reaction to form a
dibenzofuran in CDCl3 solvent. Notably, the 4-position in the dibenzofuran was deuterated.
It's true that the allyl group will migrate more easily than the methyl; I just wanted to point out that yield may depend on solvent choice.
[Edited on 12-21-2017 by Metacelsus]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
You are at the U of M, Metacelcus? Ski-U-Mah! Who is your advisor there? I was there, 25 years ago.
[Edited on 12/22/17 by PirateDocBrown]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Prof. Thomas Hoye is my advisor.
It's nice to see a fellow Gopher on Sciencemadness. Did you study chemistry while you were at the U of M?
[Edited on 12-22-2017 by Metacelsus]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
I did indeed! I got my undergrad there.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I can now post the thesis file again, since the issue has been favorably resolved.
It is attached here:
Attachment: Metacelsus Thesis.pdf (859kB)
This file has been downloaded 606 times
And a point of correction: I recently discovered that the silicon linkers described in the thesis do in fact undergo the HDDA reaction, albeit at much
higher temperatures (250 to 280 °C).
[Edited on 8-16-2018 by Metacelsus]
|
|
|