Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Deflagration of Nitrocellulose
I'm making a cannon powered by nitrocellulose, and I need to know (among other things) initial rearward impulse, exit velocity of projectile and
maximum chamber stress - as accurately as possible.
For these calculations, I need a value for the energy of combustion of Nitrocellulose, I haven't been able to find one ANYWHERE! it's really
starting to freak me out. If anyone knows where to find out, please tell me - or indeed if you happen to have it stashed away in a vault like
hippocampus, please tell me!!
aarrgh
Also, is this correct?
Nitrocellulose made by the nitration of cotton in the presence of a sulphuric acid catalyst is a 50/50 mixture of di-nitro-cellulose and
tri-nitrocellulose...
½ C<sub>6</sub>H<sub>7</sub>O<sub>2</sub>OH(NO<sub>2</sub> <sub>2</sub> + ½
C<sub>6</sub>H<sub>7</sub>O<sub>2</sub>(O<sub>2</sub>N)<sub>3</sub> <sub>2</sub> + ½
C<sub>6</sub>H<sub>7</sub>O<sub>2</sub>(O<sub>2</sub>N)<sub>3</sub>
it deflagrates to the gaseous components:
7H<sub>2</sub>O + 8CO + 2.5*N<sub>2</sub> 4C
obviously leaving a carbon residue.
Any help is greatly appreciated,
Sincerely
-Ramiel
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
First of all its cellulosenitrate, because cellulose itself has OH groups which can be nitrated resulting in O-NO2 groups.
I'm drawing the chemical structure right now and I have the formation enthalpy somewhere too.
Give me a few minutes.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
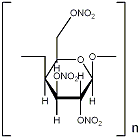
Here's the structure.
The decomposition products are CO2, CO, H2O en N2. No carbon.
Some data about cellulosenitrate:
Formation enthalpy - explosion heat - OB
13,4%N: -2390kJ/kg /\ 4409J/g /\ -29,2%
12,6%N: -2598kJ/kg /\ 3983J/g /\ -34,5%
11,6%N: -2859kJ/kg /\ 3480J/g /\ -41,2%
None of these form any carbon upon detonation. The treshold level for carbon formation is OB < -47,4% with a few exceptions.
BTW, be careful when determining the formula for this structure, I don't know if you must count in the carbon bridges or not..
This information is from JoPEP, 27, 2002
[Edited on 21-6-2003 by vulture]
[Edited on 21-6-2003 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Amazing! 
that's exactly what I was looking for!    
thanks vulture - I'll be sure to credit you in my calculations  
It'll be up in about T-2.5 hours on http://www.geocities.com/xxramielxx
[edit - no shame! blatent self linking there!]
[Edited on 21-6-2003 by Ramiel]
Caveat Orator
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
I will, suffice to say that it's quite complex!
I promise you can have a look at my site when I've finished it in another 2 hours
Caveat Orator
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
yea, but I want an exact value 
my mechanical engineering lecturer tells us that engineering is mostly useless, since we take values of massive accuracy, combine them with guesses in
orders of magnitude, and then display the answer to even greater levels of accuracy. It's in my schooling - I can't disobey my master. 
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
That sounds familiar Ramiel! Calculating the power needed for a pump using meters that were calculated from ft using 5 decimals and then saying the
friction in a 1000m pipe is neglegible....
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
haha! sure does.
Here in Western Australia, the crown's case in court was supported once by an engineer's calculation. He calculated that a car was doing
160km/h (100mph) in a 60 zone (40mph) at the time of impact. It was found using more accurate measurements, that the car was doing more like a measley
67 km/h (43mph). The jury was encouraged to ignore completely both figures.
So much for science bringing light into dark places... 'empirical data' my foot.
[edit: the maths isn't not looking good! I'm out by a factor of 10^3 somewhere I think]
[Edited on 21-6-2003 by Ramiel]
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Are you sure you aint confusing the kJ/kg and J/g figures?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Okay, here's the link:
http://www.geocities.com/xxramielxx/stuff_ive_made/math.html
you might have to copy and paste the url
It's all a bit dry, but at least you can rest your weary head knowing that I did more work for it than you did 
If you have any suggestions, feel free to go through it all with a fine tooth comb 
As for me, I think I'll rest my weary head.
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
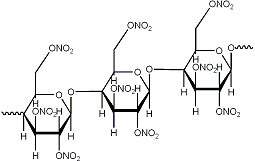
The heavily nitrated molecule (which I will no doubt create) has an additional ONO2 group instead of an H atom.
What do you mean? You can't add another ONO2 group, certainly not in the place of a H, that would be a nitro group IF possible at all.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Oh.
I can't understand now why I wrote that... 
I suppose it must be because the molecule as you drew it, sir vulture (not meaning to be rude), seems to have too little oxygen to balance the
detonation equation - maybe I'm missing something basic 
Ah well, I always was too good for those normal molecules ;\
Caveat Orator
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Ramiel, no offence taken.
That was bugging me too at first.
the problem is the bond sticking out to the left which makes you assume it's a carbon atom. It's not! It should directly connect to the
oxygen on the right side!
I've included another gif that should clear it up.
EDIT: Argh, that doesn't seem to be correct either as the cellulose is 1,4 bonded or something. Anyways I've been studying for my exam
organic chemistry all day and if I continue now I'll get my head tied up in a knot. We'll sort it out sometime...
[Edited on 21-6-2003 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Ramiel, do you use some magic script in order to get rid of the annoying pop up menu from geocities ?
Nice site design BTW !
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
Ha, the magic is the simple addition of a liberal dash of 'incompetence'. I made the entire site by hand (except perhaps the navbar...) so
there's a lot of 'non standard' coding in there. It seems when geocities tries to automatically add the code for it's crappy
little ads, it breaks it's teeth on the so called l33t coding.
Thanks
Hey Vulture, don't worry about helping me, already you've been an invaluable aid. Good luck on the exam.
ps. The ads work on most other browsers I've recently found out..
Caveat Orator
|
|
|