Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Piperidine from the hydrolysis of piperine
This thread is a report of work still in progress but was driven by my need for a small amount of piperidine and I have no recovered 34.9g of slightly
moist piperidine hydrochloride from the hydrolysis of piperine.
There are about 10 threads on the SM site on the extraction of piperine from pepper corn but only 2 on the hydrolysis and they are both useless. For
this reason I have started a new thread.
These experiment were developed from a YouTube video produced by Chem Player (https://www.youtube.com/watch?v=KDGIYEaGCZw&t=1s). He used 7g of piperine he had previously extracted from pepper corns but because the yield
of piperidine could only be about 2g at best and I was more interested in obtaining piperidine in workable quantities I chose to buy 100g of
commercial 95% piperine extract and scale up Chem Player’s method by a factor of 5, e.g. using 35g of crude piperine, for my first attempt.
The Hydrolysis
25.160g of 85% potassium hydroxide flakes were placed in a 500 ml conical flask containing a fairly large (about 45mm) stir bar. 200ml of 95% ethanol
(rectified spirit) were then added and the mixture stirred until the alkali had dissolved. 35.060g of commercial piperine extract (note 1) was then
added to the reaction mixture.
 
Photo 1 Flask with KOH in rectified spirit
Photo 2 The yellow slurry after adding the piperine
A brilliant yellow suspension formed immediately but gradually, on heating, the piperine dissolves giving a deep honey brown–orange solution. A
reflux condenser was added to the flask and it was gently heat to boiling then refluxed for 4½ hours. After about 20 minutes refluxing a crystalline
precipitate starts to form. As refluxing continued the amount of solid makes the slurry rather thick and but not difficult to stir though a large stir
bar is needed.
 
Photo 3 The dark honey brown but clear solution after 10 minutes
Photo 4 The crystalline precipitate after about 2 hours reflux
After the reflux period, the suspension was allowed to cool a little and then the condenser re-arranged for simple distillation; no temperature
monitoring was required. The liquid was distil off until the residue becomes too thick to stir, this resulted in about 170ml of distillate. When the
flask was cool enough to be handled with bare hands 70ml of water were added and the solid stirred up into a dark brown slurry then it was left to
cool to room temperature. The slurry was filtered at the pump with a 70mm Buchner funnel and the cake washed with about 30ml of the distillate and
then with about 40ml of rectified spirit to give an almost white cake. The cake was drained as dry as possible and finally dried on a watch glass. The
yield of crude potassium piperinate was 27.714g which is about a 92.8% yield though it is still rather impure (note 2).
 
Photo 5 The set-up for the removal of the solvent
Photo 6 The filtrate after collecting the first crop of K piperinate
The filtrate was returned to the flask and distilled again until the residue was again difficult to stir and then cooled (note 3). All of the
distillates were combined and rendered weakly acid with 30% hydrochloric acid, about 13ml were required. The acidified distillate was then distilled
again with temperature monitoring; the first 230ml or so distilled below 95°C and was kept for recovery of the alcohol. Another 70-80ml of
increasingly cloudy distillate passed over and was discarded; it smells distinctly of pepper and probably consists of some un-hydrolysable essential
oil in water. The small amount of slightly discoloured liquid left in the flask was transferred to a shallow bowl and evaporated to dryness with
constant stirring on a hotplate. The yield of very pale yellow piperidine hydrochloride was 12.66g (It took several attempt to get the weight this
low, the product is very soluble and deliquescent).
 
Photo 7 The crude dried potassium piperinate
Photo 8 The dried piperidine hydrochloride
Recovery of Piperinic acid
The crude potassium piperinate (27.714g) was dissolved in 200ml of hot water, cooled to a little above room temperature and filtered. The filter cake
consists of a very little off-white residual piperine and weighed 1.517g.
 
Photo 9 Dissolving the potassium salt in water - the insoluble is partly piperine
Photo 10 The filtered K piperinate solution
The clarified solution was acidified with 30% hydrochloric acid until it was strongly acid and left to stand for several hours, the result was an
almost solid butter-like pale yellow mass. The mass was slurried with a stirring rod and filtered at the pump using a 90mm Buchner (note 4) funnel and
washed with cold water. The cake was dried in a warm location for several days to obtain a constant weight; the product is a fine pale yellow powder
resembling sulphur and is quite odourless. The yield was 24.698g (theory 25.43g for a 95% starting material).
 
Photo 11 Filtering off the precipitated free piperinic acid
Photo 12 The dried filter cake of crude piperinic acid
A little potassium chloride efflorescence was observed on the final dry solid so the dried crude product was ground to a powder (it’s hard and
brittle), dispersed into 200ml of water and heated to about 70°C and cool. It was filtered and sucked dry at the pump and then air dried at 35°C to
constant weight yielding 20.301g of pale yellow powder. Attempts to recrystallize small portions of the piperinic acid with various solvents indicate
that the best solvent is pure isopropanol, about 40ml being required per gram of acid being recrystallized.
Notes
Note 1: The piperine extract was bought from an online supplier and claims to be 95%. It is a very pale yellow free flowing powder.
Note 2: An attempt was made to recrystallize the potassium salt from water and while this is easily done and the very steep solubility gradient means
recovery is high the crystals were very fine and fluffy and difficult to wash on the Buchner funnel. Some residual piperine is also present and
necessitates the filtration of the solution.
Note 3: The residue in the flask was leached with a little recovered clear methylated spirit and transferred to a filter funnel. The dark brown
filtrate was discarded as it contained much resin but also some residual piperine and with larger quantities may be worth leaching with hydrochloric
acid. The light brown cake when dried weighed 2.79g. This was stirred into about 15ml of boiling water and filtered, the small amount of light brown
residue is probably mostly unaltered piperine but was discarded (<1g), the filtrate was left to crystallise, it set almost solid but when stirred
up became a slurry that was drained with suction in a small Buchner funnel, washed with a little rectified spirit and dried. The pale straw coloured
material is crude potassium piperinate, yield was 0.870.
Note 4: with a smaller diameter funnel the volumous precipitate forms a cake too thick to wash easily.
Discussion
The next process will be the liberation of free piperidine. At the moment I plan to do this with either solid sodium hydroxide pellet or calcium
hydroxide powder. I plan to arrange a flask for distillation and then place an excess of the alkali in it with a large stir bar and then run in slowly
from a sealed dropping funnel the piperidine hydrochloride solution. The latter compound is extremely soluble in water and so the amount of additional
water to be absorbed by the alkali is actually small. Piperidine boils at 106°C without vacuum assistance but vacuum equipment may help the
disengagement of the base from the solid alkali.
I am still working up some of the residues so I am not going to discuss recoveries just yet but you can work them out yourself (I'll cover this in the
final write-up for pre-pub) but taking the 95% piperine claim into account and the fact that the final crude piperidine hydrochloride may still be a
little damp the recovery of piperidine as hydrochloride is probably about 80%, which is pretty good. Working with larger quantities than Chem Player
probably greatly reduces mechanical losses and the addition of water at the end of the distillation helps drive off the remaining piperidine too.
Further Experiments
I am current carrying out a second hydrolysis using a slightly different protocol. I this case I refluxed for 7 hours then simply cooled the slurry of
crystals to room temperature and filtered of the crystals. They were washed with about 50ml of rectified spirit on the filter and then dispersed into
200ml of fresh rectified spirit and filtered. The cake was drained as dry as possible and the dried on a large watch glass at 35°C. This gave 49.511g
of pale yellow crude potassium piperinate from 65.1g of piperine.
The filtrates were returned to the original flask and distilled until about 450-500ml had distilled off. The residue was cooled and filtered, the cake
was washed with 30ml of distillate and 30ml of fresh rectified spirit. When dried this gave a further 3.696g of crude potassium piperinate. The
filtrate smelled strongly fishy so was returned to the flask, 50ml of water added and the distillation continued until a thick tarry residue was all
that remained.
All of the filtrates were combined, acidified with 30% hydrochloric acid, about 24ml were required, and distilled in stages in progressively smaller
flasks until only a little pale yellow liquid remained. This was poured into a shallow bowl and evaporated with constant manual stirring until it
became a dry crumbly yellowish solid. Heating and stirring were continued until when a cold watch glass was place over the bowl not mist of
condensation form on the it. This gave another 22.231g of pretty dry piperidine hydrochloride, slightly darker than in the first experiment.
The potassium piperinate from this experiment can't be pure since this would represent almost 100% recovery and I recovered another 1.87g of rather
brownish K salt from the gunk left at the end of distillation by leaching it with alcohol until all of the brown tar had dissolved. I am currently
trying to purify this material.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Boffis,
Very nice piece of work. Well done and illustrated.
Piperidine distills as an azeotrope with water which is somewhat difficult to dry. I have prepared piperidine by acid hydrolysis of N-formylpiperidine
followed by concentration, basification and distillation of the reaction mixture (pH about 14). The distilled piperidine azeotrope was dried by
standing over potassium hydroxide (2 changes of KOH) for several days then redistilled.
Have fun!
AvB
|
|
|
chemplayer...
Hazard to Others
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Superb write-up and well done on such great results! You're quite right - when we did this we were focusing more on getting the piperinic acid rather
than the piperidine, and our method to distill down to dryness is somewhat clumsy and leads to a degree of loss that you overcome with scale and a
better executed procedure.
Interesting that you find the piperidine.HCl quite hygroscopic as both times we've obtained it (from piperine hydrolysis, and from sodium/alcohol
reduction of pyridine) it didn't seem to absorb moisture noticeably quickly. Interested to know if you've tried recrystallising the piperidine.HCl as
we couldn't come up with a way to do this?
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Many thanks for the comments guys.
@AvBaeyer, do you think I could dry mix in a flask the piperidine hydrochloride with say NaOH or KOH pellet by shaking them together and using the
heat of the reaction to drive off the anhydrous piperidine or do you think this might get out of hand? Might it be better to add the saturated
hydrochloride solution to a large excess of KOH pellets?
My original idea was to use excess calcium hydroxide with perhaps a few coarse lumps of calcium oxide, load the flask quickly and then gently shake, I
am pretty sure the reaction would then self perpetuate. The idea here is than the calcium chloride forms lower hydrates that require near red heat to
remove the water of hydration so the calcium salt would effectively remove the water and only dry piperidine would distil off. Again I can see that
there is no way to control the reaction once started.
@ChemPlayer My lab at this time of year is rather cold and damp, about 3-5 C at the moment. My analytical balance I keep in a small heated office and
the piperidine salt from the first batch has been in there for about 5 days and doesn't appear to have gained more than about 0.05g so it may not be
as hydroscopic as I had first imagined but it is insanely soluble in water and hangs on to the last bit of water tenaciously.
Any ideas what to try with the piperinic acid apart from making piperinal? Interestingly I noticed it is structurally related to sorbic acid too:
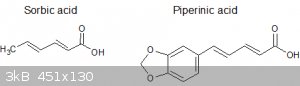
I was wondering if it will function as a diene and condense with a suitable dienophile like maleic anhydride to form a
4-phenyl-dihydrobenzene-1,2,3-tricarboxylic acid; I read somewhere that sorbic acid will take part in such reactions. I also read somewhere that
2,3-dimethylbutadiene condenses with maleic anhydride to form 4,5-dimethyldihydrophthalic acid but I can't remember the conditions or the reference.
Or is this just wishful thinking  . .
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I hydrolyzed N-formylpiperidine with excess 6M HCl, then distilled the reaction solution to a syrupy consistency. The last 50% or so of the
distillation was done under aspirator vacuum (ca. 30mm). I did not try to take it to complete dryness. Sodium hydroxide (50% aqueous) was added until
pH>14 by pH paper. The resultimg mixture was carefully distilled collecting distillate in the range 90-106. Piperidine forms an azeotrope with
water (bp 92.8°C, 65% of piperidine by mass). Solid KOH was added to the distillate (procedure repeated twice) then the liquid portion was distilled
again collectiing at 104 - 107 C. My overall yield from N-formylpiperidine was ca 80%.
AvB
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Boffis  | | @AvBaeyer, do you think I could dry mix in a flask the piperidine hydrochloride with say NaOH or KOH pellet by shaking them together and using the
heat of the reaction to drive off the anhydrous piperidine or do you think this might get out of hand? Might it be better to add the saturated
hydrochloride solution to a large excess of KOH pellets? |
If you're worried about it getting out of hand, mix the dry piperidine hydrochloride with sodium carbonate and heat it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@DraconicAcid, won't this give piperidine carbonate which I presume will dissociate to piperidine water and carbon dioxide and then recombine on
cooling?
I think AvBeayer's method sounds better, I think that I may try a very strong solution on excess NaOH pellets.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Not if the carbon dioxide vents away before recombining.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by AvBaeyer  | I hydrolyzed N-formylpiperidine with excess 6M HCl, then distilled the reaction solution to a syrupy consistency. The last 50% or so of the
distillation was done under aspirator vacuum (ca. 30mm). I did not try to take it to complete dryness. Sodium hydroxide (50% aqueous) was added until
pH>14 by pH paper. The resultimg mixture was carefully distilled collecting distillate in the range 90-106. Piperidine forms an azeotrope with
water (bp 92.8°C, 65% of piperidine by mass). Solid KOH was added to the distillate (procedure repeated twice) then the liquid portion was distilled
again collectiing at 104 - 107 C. My overall yield from N-formylpiperidine was ca 80%.
AvB |
@AvB, what's the function of the KOH at the final of the process? Is it just to sucking water and dry the piperidine azeotrope before distilling?
Could it be substituted by anhydrous K2CO3 or molecular sieves?
I'm intending to produce some piperidine and thinking about buying some N-formylpiperidine, since piperidine is closely watched here where I live.
[Edited on 23-1-2018 by Chemi Pharma]
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Chemi Pharma,
The function of the KOH is to absorb the water. Sieves might work if there is not too much water to overcome. K2CO3 will not be fully effective as
some of the piperidine will end up as the carbonate or bicarbonate. Piperidine is typically dried with sodium, calcium hydride, or KOH.
After the final distillation I stored the piperidine over KOH.
Hope this helps.
AvB
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
A word of warning.
I just bought some more 95% piperine extract from an ebay supplier in India. It claims to be black pepper extract and was darker in colour than the
previous sample so I decided to do some experiments on it to see if it could be upgrades by 1) recrystallisation of isopropanol and 2) hydrochloric
acid extraction to give a hydrochloride solution and then reprecipitate with a base.
Neither process worked and the conc sulphuric acid test for piperine failed too (no orange brown colour powder blackens and chars). The IPA extraction
left 63.5% a pale alcohol insoluble residue which is partly soluble in water and partly just swells. I would say that this material is starch or
similar polysaccharides. The green IPA extract has now evaporated to a small volume and only a little flocculant ppt has formed. If the starting
material were even 10% piperine crystals should have formed by now. A trace of an organic base is present and it can be hydrolysed to a volatile fishy
smelling amine but the amount formed is very small.
It is blatantly clear though that this material is not a piperine extract and consists mostly of starchy materials.
The extract used in the experiements above was purchased in the UK, U2U me if you want details.
|
|
|