guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Cool product from aldol condensation of acetone
I saw this mentioned in the DPPP thread but no one went over it since everyone was worried about dppp.
After reacting 30% HCl with acetone with heat for 10 hours, Na2CO3 was added to it until fizzing stopped and the color instantly changed from dark red
to clear, with some unreacted acetone floating on top.
In the water part, a white fluffy precipitate can be seen forming. Thi precipitate is insolube in acetone and water. When acid is added to it, it
can dissolve forming a yellow-orange solution, and it reverts back to the solid if base is added.
It seems to be the only pure thing I can extract from the condensation, so I'm interested.
Note that this is NOT phorone.
The fact that it has color only when it is protonated may suggest something about its structure. I think it may be a condensation of acetone with
mesitylene. Is there any way to verify this?
[Edited on 2/23/2007 by guy]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
mmm. Indicating tar!
I don't think that mesitylene a sufficiently active ring to condense with acetone. It seems more likely that (all things being equal) the acetone
would self-condense far more readily (even so, the route catalysed by alkali works better, leading to reddish brown "crap").
My guess is that your chromophore is a larger conjugated condensation/dehydration product. The reversibility suggests that the chromophore is an
ionised form, though. Very interesting, usually (at least in my experience) the compounds which are more colored when acidic involve a charge transfer
complex with a metal, viz. catechol:Fe.
But--when strongly acidic, pH<0, phenolphthalein is *orange*, so we cannot rule out the cationic form leading to some (probably via reversible
dehydration) conjugated form.
A very interesting question--have you tried to isolate this stuff (TLC, etc.)? You will need to do this before you begin characterization.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by Ozone
mmm. Indicating tar!
I don't think that mesitylene a sufficiently active ring to condense with acetone. It seems more likely that (all things being equal) the acetone
would self-condense far more readily (even so, the route catalysed by alkali works better, leading to reddish brown "crap").
My guess is that your chromophore is a larger conjugated condensation/dehydration product. The reversibility suggests that the chromophore is an
ionised form, though. Very interesting, usually (at least in my experience) the compounds which are more colored when acidic involve a charge transfer
complex with a metal, viz. catechol:Fe.
But--when strongly acidic, pH<0, phenolphthalein is *orange*, so we cannot rule out the cationic form leading to some (probably via reversible
dehydration) conjugated form.
A very interesting question--have you tried to isolate this stuff (TLC, etc.)? You will need to do this before you begin characterization.
Cheers,
O3 |
If it is a large conjugated system, then why is it white in neutral to basic conditions? A similar compound that exhibits this property (colorless in
basic, colored in acid) is trityl chloride, which is (C6H5)3CCl. When hydroxide is added, it disrupts the conjugated system. When acid is added, it
removes the hydroxyl and becomes colored again.
Mesitylene has been alkylated using aldehydes and strong acids (formic acid works too). This was done by Baeyer, 7 years before the discovery of
Friedel-Crafts reaction.
[Edited on 2/23/2007 by guy]
[Edited on 2/23/2007 by guy]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hmm,
Perhaps the trimethylation *is*sufficiently activating. Trityl chloride, however, bears no resemblence, whatsoever, to the structure that you posted
originally (many good chromophores are based on this and the color (or lack therof) results from the conjugated system). With Phenolphthalein (ln),
removing the proton leads to conjugation; further increase in pH leads to a-deprotonation which disrupts conjugation leading to decolorization.
So, in any rate, we *are* dealing with a larger, conjugated molecule. I understand that some small amount of the aromatic material would be integrated
as an equilibrium-rate determined process.
On Point,
O3
Still, I cannot fathom why the acetone would not be self condensing (kinetically favored) under the conditions when?? Acetone is in molar excess?
[Edited on 23-2-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
| Quote: | Originally posted by Ozone
Still, I cannot fathom why the acetone would not be self condensing (kinetically favored) under the conditions when?? Acetone is in molar excess?
|
It does. A very small percent forms this product, there is very little of this product compared to the rest of the stuff stuck in the unreactied
acetone. THis stiff just precipitates out when you neutralize the solution.
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
Yet I found this to be interesting so I try experimenting this a bit! FAILED!
I cannot produce the mentioned solid!
| Quote: | After reacting 30% HCl with acetone with heat for 10 hours, Na2CO3 was added to it until fizzing stopped and the color instantly changed from dark red
to clear, with some unreacted acetone floating on top.
In the water part, a white fluffy precipitate can be seen forming. Thi precipitate is insolube in acetone and water. When acid is added to it, it can
dissolve forming a yellow-orange solution, and it reverts back to the solid if base is added.
It seems to be the only pure thing I can extract from the condensation, so I'm interested. |
Here is what have been done :
- 20 mL of 99.5%+ acetone is put into the flask, slowly adding 20mL of 12.5M HCl providing the clear colorless solution which heats up a bit (26
--> 46.5 degC)
- Heat is applied at 65 (+- 5) degree celcius with stirring for 5 hours, the mixture start aldol condensation thus giving more and more intensity in
color.
Start

20 min

2 hours

5 hours no color change is observed.

Since no color change is observed, i decided to use the so-deep red liquid as the starting material. This material smells a bit lachymetry, which may
comes from high amount of HCl(g) as its contributor, but has some very distint smell undescribable in word, not quite nice smell tho.
After letting the solution cools down to 30 deg C (It's the room temperature here) solid (hydrated) Na2CO3 is then slowly added producing a hissing
noise of CO2 bubbles.


During the addition, a distint smell has formed, which is somewhat DIFFERENT smell from the starting liquor (?!) -Note1- and the
smell bursted off every time Na2CO3 is added. At first I think the heat of neutralization drives the volatile molecule out, however I found that the
temperature doesn't rise in considerate amount for this. (30 -> 33 degC) so it maybe just CO2 bubble saturating solution kicking the molecules out.
Here is the clip on neutralization:
at pH~3
http://upload.manyfile.com/get.php?1c42ca8993bae23e6a747b6e6...
at pH~6
http://upload.manyfile.com/get.php?daded7d90062766cd330dad34...
As soon as Na2CO3 is added to the solution, the precipitate started to form. I, however, think the precipitate IS NaCl, which the
solubility is very suppressed especially in the flask with hefty amount of Cl- from HCl (common ion effect)
At pH 6; the precipitate got more "heavier" consistency, getting whiter and separation is easier from the liquid, comparing to the
bubbly gooey mass at the start. I supposed this should be NaCl.


After the pH reach 8, the solution suddenly change its color to transparent light yellow

As it changes color, something must have happened here, so I filter it with Whatman 93(R) filter paper which filters easily. The
yellow liquid is then collected a few mL (~5mL) -Note2-
The liquid has a pungent odor unlike the acrid one before, And I even find it's somewhat sweety yet ketonish smell (still it should contain acetone
plus other products). It is not heavy at all and has a viscosity like water. (sorry for the blurry image, out of focus!)

While the solid collected, which I assume to be mainly consist of NaCl, is then dissolved in 30mL water. (Because it is mentioned that the product is
NOT soluble in water), instead all of them are soluble! And I'm left with a "translucent" solution (like 2-3 drops of milk in a glass of water) which
doesnt' settle after let it sit for 30 minutes. The cloudy thing I think it's from the absorbed organic matters on NaCl surface and/or defected
crystal structure, so I try adding toluene to it and bring to heavy stirring for 10 minutes. This result in separate phase of YELLOW toluene. and
clear solution of water-salt mix.

The above layer of toluene also give a hint of smell like the filtered yellow liquid (I've no wonder of this!)
.....
So, could you go further in detail on how the said water-insoluble precipitate is created? I'm more interested in this more than the existence of DPPP
itself!
Note1 : The smell cannot be describe in word, but it's a little bit "sharper" and "more acrid" comparing to the start solution. It is
not the HCl(g) kind of sharp smell, but like organic acid-resemblance sharp smell.
Note2 : the yellow liquid dispersed well to the filter paper and gives a yellow paper with nice fruity and ketonish smell after
acetone is (supposed) to evaporate all away.
Comments are welcomed!
[Edited on 2-2-2009 by Pomzazed]
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
| Quote: | After reacting 30% HCl with acetone with heat for 10 hours, Na2CO3 was added to it until fizzing stopped and the color instantly changed from dark red
to clear, with some unreacted acetone floating on top.
In the water part, a white fluffy precipitate can be seen forming. Thi precipitate is insolube in acetone and water. When acid is added to it, it can
dissolve forming a yellow-orange solution, and it reverts back to the solid if base is added.
It seems to be the only pure thing I can extract from the condensation, so I'm interested. |
So here are my questions:
- How the procedure is done in exact.
- How can the precipitate you get NOT soluble in water AND acetone? It is sure that NaCl is formed and this will dissolve in water. (but not acetone)
- Can you give the approx %yield of it (instead of "little")
- Can you filter it and recrystalise it to see if it retains the property?
If it has that mentioned property it then will be very interesting substance! Yet I still fail making this. Have seen this mentioned few times!
Thanks in advance 
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
Pomzazed
Hazard to Self
 
Posts: 57
Registered: 15-9-2008
Location: In th' Lab
Member Is Offline
Mood: Acylated
|
|
eh? can I bump this?
Althought its 2012 now I still find this interesting, as I could not produce the aforementioned 'indicator tar'
Don't stare at me making fumes... I'm just experimenting with some gas...
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
I would suggest few (little) thing about this.
I have done a simmilar experiment not long ago (with basic catalyst) and I also ended up with a brown/black tar like this, but when I added acid,
water anything it didn't get white, it stayed the same black gunk. So, You are luck
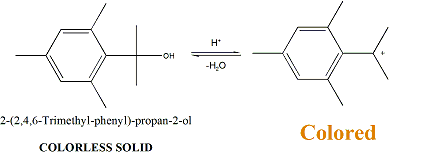
The second thing is: the 2,4,6-trimethylphely-propan-2-ol is a liquid and not a solid, it has a high (230 Celsius) boiling ponit and it is highly
viscous.
And because of it is a tertiary alcohol it could be easily dehydrated to give 2-Isopropenyl-1,3,5-trimethylbenzene what is also a highly viscous oil
with a high boiling point.
I would say that the resulted white participate from this reaction is a small molecular weight polymer...
It would be good to know that it is soluble in any solvents, if yes, is it possible to crystallize it (aromatic compounds crystallizes more easier
than non aromatic stuffs), and if it formed crystals a melting point would be also good.
And if TLC is available, also a chromatogram would be useful (to see the number of the components).
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
|