| Pages:
1
2
3 |
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
It would be interesting if you were able to make the Fluoromanganate, but that shouldn't be the only possible compound to release fluorine. By now we
are focusing highly fluorinated metals, that would release the Fluorine, which are easy to make if you already have Fluorine, so it's more or less a
fluorine storage. The same can be done with highly fluorinated Cerium for example, there is a whole list of chemicals that would do the same kind of
chemistry. And it seems good, because the Manganese one can be made without using Fluorine...if it forms like that...
The question is, are you trying to test, if Fluorine can be made at all in a home lab, or are you heading for bigger amounts? Because for the first
one, there are a couple of options to test. I'd also move from complex based reactions and possible electrolysis (I don't have a problem with
electrolysis, I thought of that as well, but the problem is you would have to pass the F2 through NaF or KF to get rid of the HF in there and this
kind of prevents a good circulation and may lead to contact between the Hydrogen and Fluorine).
The question is, either redox-based reactions or thermal ones. The latter ones are already used. There has been a recent paper, where
Copper(II)fluoride was used to fluorinate a compound and they assumed elemental Fluorine would form. It seems that with a small stream of Oxygen,
Copper(II)Fluoride would form the much more stable CuO and release Fluorine at high temperatures (500-600°C), which I think is a really interesting
thing. Same things were tried with Silversubfluoride for example. And the use of certain Phosphates to make Fluorine is really old and can be found in
the old Gmelin books. The question still arises how well they detected it back then but I think it would be worth a try to repeat these experiments
and really test them.
And the question is also how well you can influence the redox-series here. I mean you can certainly make Sodium using Magnesium, which does not work
according to the redox-potentials, but using high temperatures, and the strong bond between Mg and O you can do it. Or Oxygen does not react with
Gold, but once you add a strong complexing agent like Thiourea, some say Iodine or Cyanide, it reduces the redox-potential by a lot. And Peroxide is
not that far apart from Fluorine, which means, that if we were able to boost the potential of peroxide, we may be able to get at least traces of
Fluorine in a high temperature reaction. Peroxodisulfate is even higher than Peroxide, so let's assume we substitute the Peroxide a bit to, let's say
a P-O-O-P-type bond. P being extremely oxophilic, O being in the wrong, high energy oxidation state, perhaps we use a cyclic product, with a lot of
strain and some high temperatures close to Fluoride....it's just an idea but I really believe that if you increase the 'energy' in the system you may
be able to overcome this basic though of redox-potentials and one of the oldest works on the synthesis of Fluorine uses Sodium Metaphosphate in
combination with NaF at 650-750°C and air to make Sodium Pyrophosphate and Fluorine.
And according to this Copper paper, I guess many other oxophilic metal fluorides may work as well...
This seems like you would require a lot of exotic compounds but that was just the best combination I could think of at the moment, you can certainly
reduce a lot here and end up with similar compounds to these easy to acess Phosphates or metal fluorides (although they have to be really dry or HF
will form).
Here is a recent example:
https://pubs.acs.org/doi/10.1021/acs.inorgchem.7b01525
Although the idea dates back to 1894 I think.
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Tsjerk - The Odin sells fixed increment (not "analogue") pipettes for around $25 USD. They are quite accurate - if properly calibrated equivalent to
Gilson/Rainin.
http://www.the-odin.com/
I don't know how any splashes/fumes will affect the piston inside the barrel, but no worse than others.
I dunno, this HF business and possibly unknown high oxidation fluorine compounds worries me. It is an interesting thread to watch though...
H.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
I find it interesting if hydrochloric acid and potassium permanganate forms chlorine gas but hydrofluoric acid and potassium permanganate just
decomposes
If manganese hepoxide releases ozone then may be the reaction with hydrofluoric acid could release fluorine
It seams an oxidizer needs to be more powerful then fluorine
So if the reaction forms ozone it could form fluorine
Perbromates anyone but those can be formed by fluorine gas so even perbromates as a very strong oxidizer is still too weak only thing perbromic acid
and manganese hepoxide would be a scary combination but in thory would that combination be a stron enough oxidizer
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Well there are also Manganese Oxyfluorides....I made some and I think woelen prepared them as well, where you use the Heptoxide and Fluoride and some
acid so basically you would also end up fluorinating you Manganese first.
I don't really know what to expect from Perbromate + Mn(VII), they are both strong oxidizers and they are both highly oxidized, it's not like one of
them could be oxidized even further besides perhaps the oxygen in there but that's pretty much it.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 521
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by symboom  | I find it interesting if hydrochloric acid and potassium permanganate forms chlorine gas but hydrofluoric acid and potassium permanganate just
decomposes
If manganese hepoxide releases ozone then may be the reaction with hydrofluoric acid could release fluorine
It seams an oxidizer needs to be more powerful then fluorine
So if the reaction forms ozone it could form fluorine |
That´s why it cannot.
Fluorine is simply too strong oxidant to form when there is a way to oxidize oxygen instead.
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
If you have any interest in working with small volumes of solvent/reagent, you really owe it to yourself to get an adjustable micropipette. :-)
Control and reproducibility are miles ahead of what you could hope to get from an eyedropper or traditional pipette.
They come in capacities from a couple microliters up to 10 ml and have gotten quite cheap:
https://www.ebay.com/itm/Brand-New-Single-Channel-Adjustable...
The basic functions are:
1. Rotate the button to change the volume setting.
2. Pressing the button down/letting back up sucks up (or releases) the set amount.
3. Push down past the point of light resistance and it will eject a little air as well to clear the tip.
4. Another button ejects the (disposable) tip.
There are also variants like the tensettes:
https://www.ebay.com/itm/NEW-HACH-Tensette-19700-01-1970001-...
These guys only have ten settings (so the 10 ml version will give you 1-10 ml in even 1.0 ml increments.) The advantage is they're very fast to
select the volume on (a single twist of the plunger.)
In the bio lab they go through a ton of the disposable tips, but for non-sterile uses there's no reason you can't re-use them.
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Woelen, another preparation of potassium fluoromanganate(IV) can be found in preparation 62 from "Inorganic Laboratory Preparations" by Gert
Schlessinger in the forum library (http://library.sciencemadness.org/library/index.html ). The relevant text is reproduced below:
Eighty-nine grams of 48% hydrofluoric acid are mixed with 11ml of water in a 150ml plastic beaker and 8.9g of anhydrous potassium carbonate are added
in portions with stirring. When the effervescence has subsided, the solution of potassium hydrogen fluoride (KHF2), is cooled thoroughly in an ice
bath and 8g of powdered potassium permanganate are stirred in. Without delay, absolute ether (free from alcohol and peroxides), is added dropwise,
with good stirring, from a medicine dropper or a small burette. To avoid any possible reaction with the non-vitreous material, the ether should be run
into the center of the solution and not onto the walls of the plastic vessel. About 2ml of ether are required for the complete discharge of the
characteristic purple color of the potassium permanaganate. A brown liquid and yellow crystals of product remain at the end of the reaction; the
mixture is left for 20 minutes in the ice bath and then the clear liquid is decanted into a second plastic beaker as completely as possible in order
to avoid etching the filter flask subsequently. The brown mother liquor is reserved for the isolation of K3[MnF6]. The crystals of product are
transferred to the suction filter with about 25-30 ml of glacial acetic acid, pressed dry, washed twice more with 20ml portions of acid, and finally
with a similar quantity of acetone. The complex fluoride is dried in vacuo on filter paper. Yield = 7.5-8.0g. The product must be stored out of
contact with moist air in plastic or paraffin coated vials because water causes instant hydrolysis to MnO2, and the fluoride, itself, etches glass
with the formation of a brown deposit.
In addition, addition of potassium bifluoride to the brown supernatant liquid precipitates potassium fluoromanganate(III) on cooling.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Sounds like the wrong salt though. We want K2MnF6 not K3MnF6.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I understand from Cryolite's post that you get both salts. The yellow precipitate is K2MnF6 and the brown material is K3MnF6. The latter one is
obtained as a bonus 
I certainly will try this synthesis, it sounds more doable than the one with H2O2. I will do this experiment with KHF2 instead of K2CO3 and adjust the
concentration of HF accordingly. This is a nice thing to try next weekend.
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Quote: Originally posted by woelen  | | The more well known synthesis of K2MnF6 (and from that F2) requires the use of SbF5 and anhydrous HF, both definitely not suitable for home chemistry.
|
But to make F2 from K2MnF6 you will need SbF5 anyway, as clearly_not_atara pointet out earlier.
In this tread I already mentioned some detailed literature procedures for the H2O2 method. The descriptions there are different from your procedure.
They first mixed HF, KHF2 and KMnO4 and added H2O2 in drops afterwards. Also, large volumes are required and the precipitated amount of K2MnF6 will be
very small in comparison the the reaction volume.
I'm sceptical about the description of Schlessinger. This procedure is based on experiments from 1899 (here). The yield seems to be much smaller than with the H2O2 method (reference), however the Schlessinger procedure claims a yield of about 62 % which would be good. Also, the ratio of product weight and reaction
volume is higher, so this might be worth a try especially when working with small volumes.
[Edited on 7-2-2018 by Pok]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now remember I did this before, using the procedure in the other thread. First at room temperature, later with chilled solutions. The result was
unsatisfactory. All manganese was reduced to mangense(II), at that time I also noticed the decomposition of KMnO4 in HF. I simply have forgotten, the
results were not that interesting.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Found a ball-milling method. This one is also dependent on intercalation:
https://www.osapublishing.org/DirectPDFAccess/77388895-B0C4-...
Wt% of K2MnF6 in the product tops out at 1.1% but longer ball milling times were not tested in the paper. The product is also a phosphor, which is
kind of cool.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Any updates on this?
I am interested in synthesizing the HOF-CH3CN complex by passing F2 into a CH3CN water solution, but of course, I dont have the F2 
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have done a little more experimenting in this direction, but without success. I do not have SbF5 (and I think I also do not want that stuff around
in my house) and my experiments with KMnO4, H2O2 and HF all have been without result. Sooner or later, the purple color of the permanganate disappears
and I get an off-white solid or clear solution. The hydrogen peroxide simply reduces the manganese(VII) to manganese(II) in the HF-solution.
SbF5 (or other covalent fluorides) are really nasty stuff. they are strongly fuming compounds, which react with water, giving dense fumes of HF and
the corresponding oxide of the other elements or some intermediate oxyfluoride. This is stuff, which hardly can be handled in a home-setting. It is
beyond what I can do safely, and hence I do not wish to have SbF5, PF5 or other easily hydrolyzed fluorine compound.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I really don't understand why H2O2 is used in this prep? It's known to catalyze the decomposition of permanganate. So why would you use it for a
reaction with permanganate?
In the prep of K2MnCl6, potassium chloromanganate, no peroxide is used, but the hydrochloric acid must be "fuming", that is, concentrated hydrochloric
acid saturated with HCl gas:
https://pubs.acs.org/doi/abs/10.1021/ic50035a002?journalCode...
Presumably it should be possible to convert K2MnCl6 to K2MnF6 by treatment with silver fluoride, which is not likely to have been tested in the
literature, but many, many other chlorine compounds are converted to the corresponding fluorides by AgF. This may thus be a workable two-step route,
particularly considering that fuming HCl, no matter how concentrated, is not nearly as frightening as anhydrous HF -- and it won't eat glass!
However, dissolving the K2MnCl6 in some solvent so as to react it with AgF is a bit of a puzzle. Water is clearly out of the question.
Hydroxyl-bearing solvents in general are not acceptable. DMSO will be oxidized. DMF will probably be oxidized. Propylene carbonate and acetonitrile
stand a chance, I think. The tetramethylammonium salt is probably more stable, according to my bad understanding of which cations stabilize reactive
anions.
Another possibility is to convert MnF2 to xenon fluoromanganate by reaction with XeF2:
https://www.sciencedirect.com/science/article/pii/0022190276...
The fluorination of xenon is kind of hard, though...
[Edited on 6-8-2018 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
and if we do obtain XeF2, we can obtain the F2 gas relatively easily, right?
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
This would only bedoable in a florinated hydrocarbon sich as freon or other florinated hydrocarbons
Silver fluoride soubility
83g/100 g (11.9 °C) in hydrogen fluoride
1.5g/100 mL in methanol(25 °C)
Maybe just find what hydrogen fluoride desolves in
http://reag.paperplane.io a good site with preparations
Everything would have to be made in situ
The HF generated by an acid boric acid and silver fluoride
Generates insouble silver borate
Some sort of modification of the electrofluorination process
To work with a safe form of HF
[Edited on 7-8-2018 by symboom]
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
I looked at a number of routes to F2. I settled on the attached as this method relies on easy-to-obtain chemicals, a simple apparatus, and was
actually a laboratory-adapted method.
I didn't pursue it as I didn't see a way to produce samples. The amount of preparation needed to end-cap hydroxyl groups and all of the rest that
needs to be done to preserve samples was simply excessive.
Albert L. Henne
J. Am. Chem. Soc., 1938, 60 (1), pp 96–97
Publication Date: January 1938
Good luck in your endeavor. The scientists of SM, professional and amateurs, have made just about everything else so why not F2 or BrF3?
Attachment: 1 Page from Henne.pdf (224kB)
This file has been downloaded 319 times
[Edited on 8/8/2018 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Acually your right
There is alot isolated who would have thought
Ceasium. potassium and phosphorous
Here is a periodic table of whats been isolated
The blue is ones that have been isolated
The purple is ones that can be isolated
The orange is radioactive
The Red is dangerous

Fluorine could be proven isolated by making a fluorocarbon by electrolysis producing HF and F in situ
[Edited on 8-8-2018 by symboom]
[Edited on 8-8-2018 by symboom]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Fluorinated hydrocarbon, you say? I have about 2 gallons of Fluorinert FC-40 that I haven't found a use for. If anyone can show me a solid plan and prove their determination to actually try an experiment
using it, I may be willing to part with some. It's very expensive and rare, so I'm very selective with it.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
What process do you rip of fluorines off these flourocarbons?
If we use electrodes, would the F2 react with the electrode itself ?
[Edited on 27-8-2018 by DubaiAmateurRocketry]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
It is a carbon anode
Here a diagram
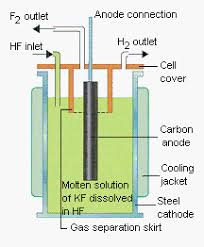
[Edited on 28-8-2018 by symboom]
|
|
|
metalresearcher
National Hazard
   
Posts: 731
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Watch out when making such a cell ! Keep the H2 and F2 STRICTLY SEPARATED !
These gases do react explosively when they come in contact with each other, they are hypergolic !
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by metalresearcher  |
Watch out when making such a cell ! Keep the H2 and F2 STRICTLY SEPARATED !
These gases do react explosively when they come in contact with each other, they are hypergolic !
|
I think that the V-shaped reactor is inherently safer by design.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Hey, here is a legit easy, HF free way for you to get F2 in a home setting, enough to smell at least. Radiation damage inside Calcium fluoride causes
defects and F2 to build up inside the crystal. When the crystal is crushed, the fluorine is released. A lot of fluorite in the environment is already
like this, due to naturally occurring uranium.
https://www.chemistryworld.com/news/fluorine-finally-found-i...
|
|
|
| Pages:
1
2
3 |