Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nitroguanylurea
When guanidine and urea nitrates are treated with ice cold concentrate sulphuric acid they are converted into the respective nitroguanidine and
nitrourea. According to old text books I have the same is true of guanylurea and diguanide. The problem is that there are three unique nitrogen
position to attach to:
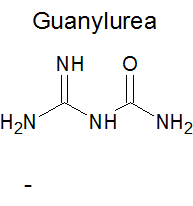
The two nitrogens at the guanyl group are effectively identical due to tautomerism, then there is the carbamyl group nitrogen the the bridging
nitrogen. The guanyl moiety is the more basic and it is likely that the nitrate ion is associated with it but this doesn't mean to say that nitramine
formation will be. The references are in old volumes of Gazetta de chimica Italia which is not readily accessible.
The dinitro compounds have been discussed on the energetics forum on occasions but not the constitution of the mononitro compound its preparation.
Any ideas about the outcome of the reaction? For comparison I have a reference that shows that the biguanide derivative can be reduced to a primary
hydrazine, so the nitro group is not attached to the bridging nitrogen.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
it' better to prepare it (Nitroguanylurea) from nitroguanidine and KNCO.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It looks like the article from Gazzetta chimica Italiana gives wrong compound, used as the substrat for nitration. Another article, from
Annalen, is cited as a source of the original procedure of nitration.
Here is a structure of the compound, given in the article from Gazzetta
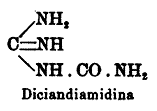
But this structure does not correspond to the compound given in Annalen under name "Dicyandiamid"(= cyanoguanidine, dicyanodiamide... ).
So, original article gives procedure of nitration of cyanoguanidine (yield 95%), not guanylurea (however it can be formed in situ from the cyanide
during hydrolysis).
It seems that procedure given by Outer may work, but it would give isomeric nitrocompound (H from guanine moiety is substituted, not
from urea as above... at least I think so)
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi kmno4, actually the term Diciandiamidina (=dicyandiamidine) is synonymous with guanylurea. As you point out dicyandiamide = cyanoguanidine. The
partial hydrolysis of of dicyandiamide converts the cyano group into an amide and generates dicyandiamidine aka guanylurea. Most strong mineral acid
will do this and yields of the appropriate salt are usually high though the monobasic acids give salts that are more manageable, the sulphate is
difficult to obtain as a dry solid with a certain composition as it crystallises as the hydrogensulphate dihydrate but this compound is always "damp"
and sticky and when "dry" is a lower hydrate though vigorous drying seem to yield a near anhydrous compound. The nitrate is very insoluble in cold
water while the perchlorate gives the best crystals but is more soluble so lower yielding.
Just to confuse matters there is also a dicyanamide which is not a dimer of cyanamide but the di cyano substituted ammonia ie HN(CN)2 it is a very
strong acid comparable to hydrochloric acid and is almost always encountered as it salts.
As to preparing nitroguanylurea from nitroguanidine and potassium cyanate; nice idea and unqualified statement but has Outer tried it or have a
reference to it preparation? In order to add a carbamoyl group you usually need to use a salt of the base with potassium cyanate. So perhaps
nitroguanylurea hydrochloride might be better (ie urea nitrate + K cyanate = biuret, however, my experience is that there are better methods). Like
kmno4 says this method has the advantage that there would be less uncertainty about the isomer produced.
The advantage of the guanylurea nitrate dehydration is that the starting materials are very accessible from commercial calcium cyanamide -->
dicyandiamide --> guanylurea nitrate. The problem is that the isomer produced is not certain and may even be a mixture.
I may try both methods and see if they are identical.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  |
As to preparing nitroguanylurea from nitroguanidine and potassium cyanate; nice idea and unqualified statement but has Outer tried it or have a
reference to it preparation? In order to add a carbamoyl group you usually need to use a salt of the base with potassium cyanate. So perhaps
nitroguanylurea hydrochloride might be better (ie urea nitrate + K cyanate = biuret, however, my experience is that there are better methods). Like
kmno4 says this method has the advantage that there would be less uncertainty about the isomer produced. |
Of course, you need an additional acid source (e.g. by using of the corresponding hydrochloride), to convert KNCO to HNCO in situ.
Nitroguanidine has one NH-group, attached to NO2 (which is practically non-basic) and 2 identical NH2 groups. The latter NH2-groups will react with
HNCO, giving a single isomer.
I do not have a reference, you can search similar reactions of HCNO with other guanidine derivatives.
[Edited on 13-2-2018 by Outer]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have just carried out the preparation of nitroguanylurea from guanylurea nitrate using basically the same method as that described elsewhere on this
site for the preparation of nitroguanidine from guanidine nitrate. The first stage appears to have worked well and I now have a reasonable amount of
moist white curds. The problem is that they are contaminated with residual sulphuric acid and so will not dry. I attempted to recrystallise a small
amount from water (1gm) but it is very insoluble even in boiling water. It is equally insoluble in alcohol (rectified spirit) and isopropanol. Any
suggestions as to a better solvent?
I intend to purify it by dispersing it in water and adding a little sodium bicarbonate and then filtering off the insoluble nitro compound.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Well the preparation of nitroguanylurea from guanylurea nitrate seems to have worked succesfully with a final yield of 88%. Unfortunately I was hoping
to compare this compound with one prepared from nitroguanidine and potassium cyanate. I carefully purified the potassium cyanate (commercial
laboratory grade material) using the procedure in Armarego & Chai. The yield was only 38% !!
The problem is that nitroguanidine is not basic enough to form salts even with hydrochloric acid so the solution of nitroguanidine with a molar
equivalence of hydrochloric acid was so acidic that the cyanate was rapidly degraded and no guanyl urea compound formed. I have another batch running
now using acetic acid but no nitroguanylurea appears to be forming, it is very insoluble and even a small yield would be an obvious ppt.
I can't find any references to any of the possible mono-nitroguanylurea so I have nothing to compare my product to.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
For anyone that is interested I found a paper, possibly the one refered to about by kmno4, in Annalen by Thiele and one of his team (1898 Annalen
v303, p107-114) in which they note that when the compound is boiled in suspension it break down into guanidine, carbon dioxide and nitrous oxide. The
indicates that the nitro group enters the compound by forming a nitramine with the H2NCO- group. This means that it has this structure:
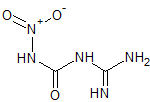
Problem solved!
[Edited on 7-7-2018 by Boffis]
|
|
|