| Pages:
1
2 |
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Essential Chemicals for the Organic Lab
I know similar lists have been made before, but I felt like making a list of what I consider to be the ideal array of reagents for an amateur organic
chemist. This is not intended to be a list of things that you NEED to have, but rather a list of chemicals that you should strive to obtain if you
wish to be prepared to carry out virtually any organic transformation. I'm probably forgetting some– if you have any ideas to add to it, let me know
and I'll add them. Keep in mind though that this is intended to be a list of chemical reagents that can be used for a wide variety of reactions, so
just because something is readily available doesn't mean it belongs on the list (citric acid, for instance). I posted this list on my blog with a
color code to show the availability of different chemicals. I'll post the list here too but it won't be color coded, so I recommend checking out the
one on my blog. Anyway, here's the list:
https://texium.wordpress.com/2018/03/06/essential-chemicals-...
Acetic acid
Acetic anhydride
Acetone
Acetonitrile
Acetyl chloride or acetyl bromide
Acetylsalicylic acid (solely to make salicylic acid)
[Alkyl]silyl chloride(s)
Aluminum metal
Aluminum chloride (anhydrous)
Ammonium chloride
Aluminum isopropoxide
Ammonium persulfate
Aniline
Anthranilic acid
Aqueous ammonia
Argon
Benzaldehyde
Benzene
Benzoic acid
Benzophenone
Benzyl alcohol
Benzyl chloride or benzyl bromide
Borane-tetrahydrofuran
Boric acid
Bromine
Bromobenzene
n-Bromobutane
n-Butanol
t-Butanol
n-Butyllithium
Calcium chloride (anhydrous)
Carbon dioxide (dry ice)
Chloroacetic acid
m-Chloroperoxybenzoic acid (mCPBA)
Copper(I) bromide
Copper(I) chloride
Copper(II) chloride
Copper(I) cyanide
Copper metal or copper(II) sulfate (for making other copper compounds)
Cyanuric chloride
Cyclohexanol or cyclohexanone
Dichloromethane (DCM)
Dicyclohexylcarbodiimide (DCC)
Diethyl ether
Diisobutylaluminum hydride (DIBAl-H)
4-Dimethylaminopyridine
Dimethylformamide (DMF)
Dimethylsulfoxide (DMSO)
Distilled water
Ethanol
Ethyl acetate
Ethyl iodide
Ethylene glycol
Formaldehyde (as paraformaldehyde or formalin)
Formic acid
Gallium metal
Glycerol
Heptanes, hexanes, or other aliphatic petroleum ether
Hydrazine sulfate
Hydrobromic acid
Hydrochloric acid
Hydrogen peroxide
Hydroquinone
Iodine
Iron metal
Lead (II,IV) oxide
Lithium aluminum hydride (LAH)
Lithium metal
Magnesium metal
Magnesium sulfate (anhydrous)
Manganese dioxide
Mercury(II) chloride
Mercury metal
Methanol
Methylamine hydrochloride
Methyl iodide
Monosodium glutamate
Naphthalene
N-bromosuccinimide
Nicotinic acid (niacin) (solely to make pyridine)
Nitric acid (azeotropic)
Nitric acid (white fuming)
Nitrobenzene
Oleum
Oxalic acid
Oxalyl chloride
Oxone
Palladium and/or platinum on carbon
Phenol
Phenolphthalein
Phosphoric acid (concentrated)
Phosphorus (red)
Phosphorus pentachloride
Phosphorus pentoxide
Phosphorus tribromide
Phthalic anhydride
Phthalimide
Piperidine
Potassium carbonate
Potassium chloride
Potassium chlorochromate
Potassium dichromate
Potassium fluoride
Potassium hydrogen phthalate
Potassium hydroxide
Potassium iodate
Potassium iodide
Potassium nitrate
Potassium periodate
Potassium permanganate
Potassium t-butoxide
2-Propanol
Propylene glycol
Pyridine
Salicylic acid
Sodium azide
Sodium bicarbonate
Sodium borohydride
Sodium bromide
Sodium carbonate
Sodium chloride
Sodium cyanide
Sodium dithionite
Sodium hydroxide
Sodium metabisulfite
Sodium metal
Sodium nitrite
Sodium persulfate
Sodium sulfate (anhydrous)
Sodium sulfide
Sodium sulfite
Sodium tetrafluoroborate
Sodium thiosulfate
Styrene
Succinic acid
Succinimide
Sulfur
Sulfuric acid (concentrated)
Sulfuryl chloride
Tetrabutylammonium bromide or iodide
Tetrachloroethylene
Tetrahydrofuran (THF)
Thionyl chloride
Tin(II) chloride
Tin(IV) chloride (anhydrous)
Tin metal
Toluene
p-Toluenesulfonic acid
p-Toluenesulfonyl chloride
Trichloroisocyanuric acid (TCCA)
Triethylamine
Triflic acid
Triphenylphosphine
Urea
Xylenes
Zinc chloride (anhydrous)
Zinc metal
[Edited on 3-7-2018 by Texium (zts16)]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Man that is some list. There is a lot that you could do with that.
I think this is my list for Santa now.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
I currently only have 10% of the chemicals on that list. Some of the harder to acquire ones require hours of syntheses and preparations (to me that
is), so in order to compile these chemicals probably would take me at least a year. Wow.
The philosophy of one century is the common sense of the next.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wow ! I got 37 / 155
Admittedly 1 of them is Water 
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Isn't this list very ambitious for an amateur home lab? I would say that this list is for a very well-equipped advanced amateur lab, the average
amateur only will have access to a fraction of these chemicals.
This is exactly the reason why I do inorganic chemistry mostly. For organic chemistry at a decent level you need a lot of fairly special and hard to
obtain reagents, which only can be used for a very specific type of reaction. In inorganic chemistry it is easier to have a decent set of fairly
general reagents, where each reagent can be used for a multitude of experiments.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
No wonder that I'm a useless organic chemist,
I have less than half of those chemicals, 58 plus a few that I could quickly synthesise.
But I say its not what you've got but what you do with it that's more important
- that's what I told the women in my life 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I appreciate the effort you've taken to write this list but I don't agree with the inclusion of many items or their colour classifications (on your
blog). I've been doing organic chemistry my whole career and have never needed to use many of those chemicals (nor do I envisage doing so), depsite a
wide variety of chemistry being covered. I don't think you can claim this to be an "essentials" list, nor should anyone looking to pursue organic
synthesis rush out to buy (or make) these chemicals.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by woelen  | | Isn't this list very ambitious for an amateur home lab? I would say that this list is for a very well-equipped advanced amateur lab, the average
amateur only will have access to a fraction of these chemicals. |
Yes, it is meant to be a very ambitious list. I don't have access to all of those chemicals. I haven't actually counted how many I have, but I
probably only have about half, with the ability to make maybe another quarter of them (which would take a lot of time). It isn't meant to be a list of
all chemicals you need if you want to do organic chemistry, and I don't want to scare anybody! It's supposed to represent all the chemicals you would
need if you want to be able to do virtually any organic synthesis. If you had all these chemicals stocked and a good selection of apparatus (perhaps
that will be my next list) you'd have lifetimes worth of experiments at your fingertips!
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DJF90  | | I appreciate the effort you've taken to write this list but I don't agree with the inclusion of many items or their colour classifications (on your
blog). I've been doing organic chemistry my whole career and have never needed to use many of those chemicals (nor do I envisage doing so), depsite a
wide variety of chemistry being covered. I don't think you can claim this to be an "essentials" list, nor should anyone looking to pursue organic
synthesis rush out to buy (or make) these chemicals. |
If you don't mind, could you be specific about some of
the ones that are most glaringly wrong in your opinion?
I've only been doing organic synthesis in a somewhat professional capacity for about a year. I'm probably heavily influenced by the specific things
that I have been working on, so you clearly have a much more complete picture than I do.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Based mainly on my electronics hobby;
. As different areas within the hobby are explored, different stuff is required
. I can't afford to fully stock an electronics or chemicals warehouse
. even stock that I have deteriorates over time
So a few basic necessities are required for ah-hoc experiments,
but mostly I think that it is best to get what you need just before you need it.
... if you can.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
It can be a real challenge to recommend chemicals; so much depends on what you're doing. But, as a starting point for a hobby lab I would say most
people will likely want/need:
Acids:
Acetic acid (glacial)
Hydrochloric acid
Sulfuric acid
Nitric acid (bought or made)
Bases:
Sodium hydroxide
Sodium bicarbonate
Strong ammonia solution
Oxidizers:
Potassium permangenate
Hydrogen peroxide (35% is readily available in the US.)
(Bichromates are useful, but due to toxicity concerns I would leave them off the list for a beginner.)
Solvents:
Distilled water
Hexane (a light solvent for extractions)
Dichloromethane (dense solvent for extractions)
Methanol
Ethanol
Isopropanol (All three common alcohols might seem like overkill, but they're cheap and easy to get.)
Acetone
...and at least one polar aprotic solvent like diethyl ether, ethyl acetate, DMF, THF, DMSO.
Reducing agents:
Lithium Al hydride and/or sodium borohydride
(Aluminum amalgams are neat, but I can't support the use of mercury in a hobby lab.)
Misc:
Iodine
Magnesium
Sodium chloride
Potassium nitrate (to make your own nitric acid if needed)
Ammonium nitrate (for recrystallization practice and some small demonstrations like an ammonia generator.)
After that, I would say 'what do you want to do?' Labs can have radically different stock on hand depending on their interests.
Of course, let's not forget the important safety stuff like Safety Data Sheets and a good fire extinguisher. :-)
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
@Reboot: That's a nice list of chemicals for an organic beginner. I'd only disagree on the LAH/NaBH4 since they are expensive and hard to come by (and
for LAH, dangerous). I still don't have either of those, and I think aluminum isopropoxide is the most accessible reducing agent of that sort for
amateurs.
My list was not supposed to be a minimum list by any means, but rather a list of what you'd need if you wanted to be able to do everything
|
|
|
Reboot
Hazard to Others
  
Posts: 141
Registered: 8-8-2017
Member Is Offline
Mood: No Mood
|
|
Interesting! In my experience hydrides have been fairly common/readily available, while aluminum isopropoxide has been a more exotic reagent.
On the other hand, it's apparently lot easier to make Al isopropoxide in the lab than hydrides, so that's certainly an advantage for a determined
do-it-yourselfer.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It's definitely less commonly seen, and it isn't as versatile as the hydride reagents, but it is trivial to make in a home lab if you have mercury,
iodine, or gallium to use as a catalyst (see multiple threads on here about it). Most importantly- it's dirt cheap.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Don't be jealous guys (lol) . Below I list my atual chemical reagents stuff. All
of them stored inside a weel exhausted and refreshed aparted room, back the LAB, at cemented shelfs, in the dark and monitored by IP camera. Don't
need to say all security rules to store chemicals were followed. . Below I list my atual chemical reagents stuff. All
of them stored inside a weel exhausted and refreshed aparted room, back the LAB, at cemented shelfs, in the dark and monitored by IP camera. Don't
need to say all security rules to store chemicals were followed.
My desire list includes some sodamide, cyanuric chloride, diethyl sulfate and ethylamine I will buy soon, cause are hard to make in the home lab.
ACETALDEHYDE (refrigerator)
ACETIC ANHYDRIDE
ACETONE
ACETONITRILE
ACETOPHENONE
ACTIVE CARBON (POWDER)
ALANINE
ALLYL CHLORIDE
ALLYL HEPTANOATE
ALUMINUM CHLORIDE (6 H2O)
ALUMINUM CHLORIDE (ANHYDROUS)
ALUMINUM POWDER
ALUMINUM OXIDE
AMINOBENZOIC ACID (PARA)
AMINOBUTYRIC ACID (GABA)
AMMONIUM ACETATE
AMMONIUM BROMIDE
AMMONIUM CHLORIDE
AMMONIUM FLUORIDE
AMMONIUM HYDROXIDE 24-26% - 28-30%
AMMONIUM MOLYBDATE
ANTIMONY OXIDE
ASCORBIC ACID
BENZALDEHYDE
BENZENE
BENZYL ALCOHOL
BENZOPHENONE
BENZOYL CHLORIDE
BORIC ACID
BROMIDRIC ACID 48%
BROMOTIMOL BLUE
BUTANODIOL-1.4
CAFFEINE
CALCIUM ACETATE
CALCIUM CARBONATE
CALCIUM CHLORIDE (ANHYDROUS)
CALCIUM HYDROXIDE
CALCIUM HYPOCHLORITE 65%
CALCIUM OXIDE
CALCIUM PYRUVATE
CHLOROACETAMIDE
CHLOROACETIC ACID
CHLOROANILINE (PARA)
CHLOROBENZENE
CHLOROFORM
CHROMIC ANHYDRIDE
CINAMALDEHYDE
CINAMYL ALCOHOL
CITRIC ACID
COBALT CHLORIDE II (6H2O)
COPPER (POWDER)
COPPER ACETATE II (1H2O)
COPPER CYANIDE
COPPER SULFATE (5 H2O)
CYCLOHEXYLAMINE
DIBASIC SODIUM PHOSPHATE (12 H2O)
DIETHYL ETHER 99.5%
DIMETHYL CARBONATE
DIMETHYLSULFOXIDE 99% - DMSO
DIMETHYLFORMAMIDE-DMF
ETHYL ACETATE
ETHYL ALCOHOL ABSOLUTE 99.8%
ETHYL BROMIDE
ETHYLENE GLYCOL
EUGENOL
FLUOBORIC ACID 50%
FORMALDEHYDE
FORMIC ACID 85%
FURFURAL
GLACIAL ACETIC ACID
GLYCERIN 99.5%
GLYCINE
GLYOXYLIC ACID
TIN ( GRANULATED - 20 MESH)
HELIONAL
HEXAMETHYLENETHETRAMINE - HEXAMINE - HMTA
HEXANE
HYDRAZINE SULFATE
HYDROCHLORIC ACID (31-33%) (37%)
HYDROGEN PEROXIDE 50% - 200 VOL
HYDROQUINONE
HYDROXYLAMINE CHLORIDRATE
INDOL 99%
IODINE
IRON (POWDER)
IRON CHLORIDE III (6 H2O)
IRON CHLORIDE III (ANHYDROUS)
IRON SULFATE II (7 H2O)
ISOAMYL ALCOOL
ISOPROPYL ALCOHOL
LACTIC ACID
LACTOSE
LEAD ACETATE II (3H2O)
LITHIUM CHLORIDE
LUGOL'S IODINE 5%
MAGNESIUM (RIBBON)
MAGNESIUM SULFATE (ANHYDROUS)
MANGANESE NITRATE (WATER SOLUTION 50%)
MANGANESE SULFATE
MERCURY 99.99%
METHYL ALCOHOL 99.8%
METHYLAMINE (40% WATER SOLUTION)
METHYLENE CHLORIDE
METHYL-ETHYL-KETONE - MEK
METHYL ANTRANILATE
METHYL ORANGE
MINERAL OIL (LIQUID VASELINE)
MONOBASIC SODIUM PHOSPHATE (1 H2O)
MORPHOLINE
NICKEL ACETATE II (4H2O)
NICKEL CHLORIDE (HEXAHYDRATED)
NITRIC ACID (53%) (65%)
NITRO-BENZOIC ACID (PARA)
NITROETHANE
N-PROPYL ALCOHOL
OXALIC ACID (2 H2O)
PARAFORMALDEHYDE
PETROLEUM ETER (30 - 60 ºC)
PHENOL
PHENOLPHTALEINE
PHENYL ACETALDEHYDE
PHENYL ETHYL ALCOHOL
PHENYL PROPYL ALCOHOL
PHENYLALANINE (D)
PHENYLALANINE (DL)
PHENYLETHYL PHENYLACETATE
PHOSPHORIC ACID 85%
PHOSPHORUS (RED POWDER)
PHOSPHORUS PENTOXIDE
POTASSIUM BISULFATE
POTASSIUM BROMATE
POTASSIUM BROMIDE
POTASSIUM CARBONATE
POTASSIUM CHLORIDE
POTASSIUM FERROCYANIDE (3 H2O)
POTASSIUM HYDROXIDE
POTASSIUM IODIDE
POTASSIUM NITRATE
POTASSIUM PERMANGANATE
POTASSIUM PERSULFATE
POTASSIUM THIOCYANATE
PROLINE (L)
PROPIONIC ACID
PROPYLENE GLYCOL
PYRIDINE
PYROCATHECOL
SALICYLIC ACID
SILVER NITRATE
SODIUM (METAL PIECES IN MINERAL OIL)
SODIUM ACETATE (3 H2O)
SODIUM BENZOATE
SODIUM BICARBONATE
SODIUM BISULFITE
SODIUM BOROHYDRIDE
SODIUM CARBONATE
SODIUM CHLORATE
SODIUM CHLORIDE
SODIUM CYANIDE
SODIUM DICHROMATE
SODIUM DITHIONITE
SODIUM FLUORIDE
SODIUM HIPOPHOSPHITE (MONOHYDRATE)
SODIUM HYDROXIDE
SODIUM HYPOCHLORITE 10-12%
SODIUM METOXIDE (METHANOL SOLUTION 30%)
SODIUM NITRATE
SODIUM NITRITE
SODIUM PERBORATE
SODIUM PERIODATE
SODIUM SULFATE (ANHYDROUS)
SODIUM SULFIDE
SODIUM THIOSULFATE (5 H2O)
STEARIC ACID
SUCCINIC ACID
SULFAMIC ACID
SULFUR (POWDER)
SULFURIC ACID (95-99%)
TARTARIC ACID- L (+)
TCCA
TERT-BUTILAMMONIUM BROMIDE - TBAB
TERT-BUTYL ALCOHOL
TETRAHYDROFURANE - THF
THIOGLYCOLIC ACID 97%
THIOUREIA
TIN CHLORIDE II
TIONYL CHLORIDE
TOLUENE
TOLUENE SULFONIC ACID (PARA)
TOLUIDINE
TOSYL CHLORIDE
TRIBASIC SODIUM PHOSPHATE (12 H2O)
TRICHLORETHYLENE
TRIETILAMINE - TEA
TRIFENYLPHOSPHINE
TRIFLUORACETIC ACID 99.8%
UNDECYL ALDEHYDE
UREA
VANILLIN
XYLENE
ZINC (POWDER)
ZINC CHLORIDE (ANHYDROUS)
ZINC OXIDE 99%
  
[Edited on 7-3-2018 by Chemi Pharma]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Texium (zts16)  | | @Reboot: That's a nice list of chemicals for an organic beginner. I'd only disagree on the LAH/NaBH4 since they are expensive and hard to come by (and
for LAH, dangerous). I still don't have either of those, and I think aluminum isopropoxide is the most accessible reducing agent of that sort for
amateurs. |
You have both of those items on your list! If the issue you take with them is safety, then bear in mind that Reboot did not specify them for
a "beginner", but for "someone looking to start a hobby lab". The two are not necessarily synonomous.
Quote: Originally posted by Texium (zts16)  |
My list was not supposed to be a minimum list by any means, but rather a list of what you'd need if you wanted to be able to do everything
|
Attempts to write such a list is futile. You cannot do everything with such a small sample of available reagents, and to expand the list
makes it meaningless and unwieldy. When you come across a reaction with a reagent you don't have, you have to place an order for it, or make it
yourself. Sulaiman picked up on this point perfectly with his analogy to stocking an electronics warehouse.
Quote: Originally posted by Texium (zts16)  | If you don't mind, could you be specific about some of the ones that are most glaringly wrong in your opinion?
I've only been doing organic synthesis in a somewhat professional capacity for about a year. I'm probably heavily influenced by the specific things
that I have been working on, so you clearly have a much more complete picture than I do. |
It really depends on how you wish to define "essential". I take that to mean the bare minimum you need to buy, excluding stuff you can easily
make yourself. For example, I would not include sodium carbonate in such a list, because the anhydrous solid can be made easily from sodium
bicarbonate, and alternatively by "neutralisation" of bicarbonate with hydroxide (in solution).
Conversely, NaBH4 is certainly essential - It is a very versatile reagent that can be tuned using additives, or converted to other useful species
ex-situ. As such, it can be used to perform most reductions required by an organic chemist. Those not effected by NaBH4 or a related species
will usually be compliant under dissolving metal conditions.
There are many things on your list that are substrates for specific reactions that you may have done, or seen done on Sciencemadness, but which
otherwise are not used all that much in "the real world". Examples include aniline, anthranilic acid, acetylsalicylic acid, tetrachloroethylene etc.
These should be omitted as they are not essential per se, and are really dependent on what chemistry you are pursuing (at a given point in time). That
is not to say you cannot do useful things with them (and this forum is full of examples of that), but that they are not themselves required for a
functioning organic laboratory as you propose.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Chemi Pharma  | | Don't be jealous guys (lol). Below I list my atual chemical reagents stuff. All of them stored inside a weel exhausted and refreshed aparted room,
back the LAB, at cemented shelfs, in the dark and monitored by IP camera. Don't need to say all security rules to store chemicals were followed.
|
Ho-Lee-Shit ... what a fabulous chem. store - I'm so jealous. 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'd probably add trifluoroacetic anhydride, TEMPO, and boron tribromide. Oh and also bipy!
[Edited on 7-3-2018 by JJay]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
What means "bipy" @JJay?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
To me essential is only what I need for my next experiment.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
BaFuxa
Hazard to Self
 
Posts: 61
Registered: 18-9-2017
Location: Mars
Member Is Offline
Mood: Buzzing
|
|
Another information of interest would be what in what quantity/ volume you get your reagents as it is sometimes hard to estimate how much you will
actually need.
Potential counts for nothing until realized.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Bipy is bipyridine:
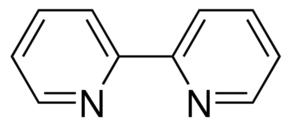
It has many uses. In particular, it can form a shelf-stable complex with chromium (VI) oxide peroxide.
Come to think of it, it is probably more useful to inorganic chemists than organic chemists, but it's still good to have around I think.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by BaFuxa  | | Another information of interest would be what in what quantity/ volume you get your reagents as it is sometimes hard to estimate how much you will
actually need. |
My (and several others here) problem is that most chemicals are via post/courrier, with attendant costs,
and suppliers need to make a larger percentage profit on small quantities to make their business worthwhile,
so where I need say 10g, 25g to allow a second run, or have a little spare,
I end up buying 500g as the price difference is so small.
This increases the required storage space dramatically.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  |
My (and several others here) problem is that most chemicals are via post/courrier, with attendant costs,
and suppliers need to make a larger percentage profit on small quantities to make their business worthwhile,
so where I need say 10g, 25g to allow a second run, or have a little spare,
I end up buying 500g as the price difference is so small.
This increases the required storage space dramatically. |
I agree. There are a lot of things which I would say I "have at my disposal", but I don't have a bottle of it anywhere. Nitric acid is a good example.
I have ammonium nitrate and potassium nitrate both, usually. If I need nitric acid, I make some. Because it doesn't take much time, isn't very
difficult, set-up/clean-up is easy, and all of this is better to me than storing nitric acid in my already packed lab space.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Are alkyl lithium compounds really beyond the reach of the amateur? I'm pretty sure making them is just a matter of reacting alkyl halides with
lithium in ether at cold temperatures under inert gas, filtering, and titrating... it's not exactly trivial, but I think some of us could do it....
|
|
|
| Pages:
1
2 |