Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Column chromatography of acid-sensitive compounds
I am currently trying to prepare a compound of the type shown below:
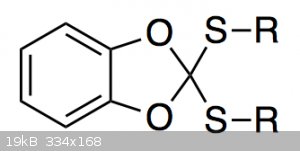
I can make it just fine, but purifying it is annoying because it slowly decomposes in contact with silica gel. The main decomposition product is
catechol carbonate.
I've heard adding 1% triethylamine to the eluent (which is 10% ethyl acetate in hexanes) might help. Alternatively, switching to alumina instead of
silica could work. However, I don't have alumina TLC plates so I can't do TLC using alumina. Distillation and crystallization are out of the question,
since the compound is a liquid that is also sensitive to heat.
Does anyone here have experience with purifying acid-sensitive compounds by chromatography? What do you think I should do?
[Edited on 3-17-2018 by Metacelsus]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I haven't done a lot of chromatography, but I suspect that alumina is generally more acidic than silica. Maybe calcium carbonate would work?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I was thinking "basic alumina, Brockman grade I." For example: https://www.sigmaaldrich.com/catalog/product/sigald/199443 Note that it says the pH of a 5% suspension in water is 9.5
Judging by periodic trends, I would expect aluminum oxide to be less acidic than silicon oxide, all else equal. However, the acidity of alumina vs.
silica gel would probably depend on the method of preparation.
I haven't ever heard of calcium carbonate being used as a stationary phase, but I will look into it.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Why haven't you already try to add triethylamine to the eluent? That's the first thing I think about, and you mentioned this option first too.
|
|
|