| Pages:
1
..
10
11
12
13
14
..
23 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Boffis  | On the point of cyanuric acid; no but I can't think of a method of testing for it apart, perhaps, from its ability to form insoluble salts with Ag or
Pb but in the presence of so much chloride from the initial reactant Pb could not be used and cyanamide reacts with ammonical Ag nitrate so a ppt
would probably be inconclusive.
Does anybody know of a test for cyanuric acid? There is a book dating from about 1930 by a German chemist called either Feigl or Angers that is full
of useful spot test for organic chemicals if anyone has a copy or know the title. These two chemist later collaberated on a classic text book on
inorganic spot test a little later. |
Calcium cyanurate is virtually insoluble so adding calcium chloride would be a good spot test there. Also the sodium cyanurate should have increased
solubility over the free cyanuric acid, although the solubility of either one is not great, and a hot water titration might identify cyanuric acid by
neutralization equivalent revealing the tri-acid character of the cyanuric acid for an aliquot as related to its mole weight. Hydrates exist for
cyanuric acid and its salts which will have bearing on quantitative weight calculations for the solid samples. You could also see what is the heat
response for the cyanuric acid as heat should depolymerize and volatilize cyanuric acid as cyanic acid which may sublime and repolymerize again as a
condensate on the cooler part of test tube. Heating the sodium salt should result in a thermal decomposition product testing postive for sodium
cyanate.
[Edited on 27-10-2011 by Rosco Bodine]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Rosco
Thanks for this information I will try it out. I have a little cyanuric acid byproduct of some work I did a while back, enough to try this out I
thing.
By the way I have just looked on Amazon and the book I was thinking of is: Spot tests in organic analysis by Fritz Feigl (Hardcover - 1960)
5 used from £9.91
I've just bought one for GB pounds 13; If anyone is interested Ièll let you know how good it is!
|
|
|
almaz
Harmless

Posts: 12
Registered: 21-12-2010
Location: Russia
Member Is Offline
Mood: No Mood
|
|
I prepared mercury nitrotetrazolates in it method:
1 solution. Sodium nitrite 3g and 1,6g copper sulphate nave in 10ml the waters.
2 solution. 1,5g ATZ, 0,06g copper sulphate in 15,45ml 58% nitric acid + 7ml water. The solution 2 I dripped to solution 2 and cooling at current 1,5
hours. The spume, oxides of the nitrogen and microdetonates did not observe. After 40 minutes the mixture became thicken. On completion of the else 15
minutes and dripping 17ml 58% nitric acid + 7ml waters, via one hour the copper salt sediment has washed at 7ml 58% nitric acid + 35ml waters. The
sediment neutralized sodium hydroxides and boiled it 30 minutes. At not it filtrated, copper oxides washed at 15ml of boiling waters. In solution the,
I dripped acid up to PH=4, evaporation of water and cooling give crystals. For clear has dissolved the crystals in 50ml boiling acetone. It filtered
and vaporized the solvent. This give the painted crystals.
The receprion to mercury salt:
0,1g sodium nitrotetrazolates dissolved in 0,7ml waters + 0,1ml nitric acid. And when heating in not water flowed the solution, contacting 0,15g
nitrate mercury dissolved in 0,5ml waters + 0,01ml acid. After slow cooling fall out the heavy crystals of beige colour, reminding croups.
Has Elsa got silver salt - they the most small white needles.
The Minute quantity mercury salt - has overpunched in 3mm glass hole. From action of the blaze immediately detonates.
Photo:
0,051-5-aminotetrazol
0,053-mixture before filrering
0,058-copper nitroterazolates
0,059-mixture on water bath
0,056-sodium salt.
[Edited on 17-11-2011 by almaz]
Attachment: phpDRWrRu (28kB)
This file has been downloaded 1278 times
Attachment: phpU4mYwb (26kB)
This file has been downloaded 1159 times
Attachment: phpVVJMW7 (30kB)
This file has been downloaded 1126 times
Attachment: phpGmfwji (25kB)
This file has been downloaded 1260 times
Attachment: php02kDij (33kB)
This file has been downloaded 1150 times
Attachment: phpLmt5id (28kB)
This file has been downloaded 1214 times
Attachment: phpi11iXH (26kB)
This file has been downloaded 1103 times
Attachment: phpVY2gIs (30kB)
This file has been downloaded 1034 times
Attachment: phpQDhFbr (25kB)
This file has been downloaded 1139 times
Attachment: phpIg7dBr (33kB)
This file has been downloaded 1169 times
|
|
|
almaz
Harmless

Posts: 12
Registered: 21-12-2010
Location: Russia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by almaz  | 2g aminotetrazole has
dissolved in
19,6ml 15 % of a solution
NaOH. Heated up to
100с also poured the small.
In the portions 2,7g KMnO4 to
Dark coloring
Liquids (dark green).
Observed strong boiling up
liquids. Upon termination of
Additions has flowed for
Surplus removals
Permanganate 0.5ml C2H5OH.
After hour heating
At 100c, hot quickly
Filtered from big a stake -
ва MnO2, the deposit has
washed out on
The filter 2х25ml
distilled boiled water and all
The filtrate evaporated to 1/3
Initial volume. After
Coolings drop out the yellow
crystals 2,9g (~70% ) sodium azotetrazolate |

|
|
|
almaz
Harmless

Posts: 12
Registered: 21-12-2010
Location: Russia
Member Is Offline
Mood: No Mood
|
|
dry weigh -2,1g (88% theory)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Well, another oxidant that works great for oxidizing sodium aminotetrazolate to sodium azotetrazolate is potassium persulfate (K2S2O8). I would prefer
this over the messy KMnO4, and alcohol is not needed to destroy any excess either. No filtering of the MnO2 is required either. I've tried it with
this persulfate and it worked, but I didn't characterize the yield so good because it was in the microscale, the yield is probably around the same
like by permanganate. The sodium azotetrazolate made from K2S2O8 reacted to open flame the same way as the one made from KMnO4, there was also no
reaction from hammer slamming on an iron plate. The appearance was the same also.
I also stated in the previous document ethanol can be used to wash sodium azotetrazolate, this is true as long as no preparation liquid liquor is
present, otherwise a white compound (no apparent energetic properties after isolation) begins to precipitate.
To any who might have wondered, there was some inaccuracies of weighing and amounts in the previous document, which is why I requested it be deleted.
Mercury diazoaminotetrazolate is actually similar in brisance as the silver and copper salt, which are similar to silver fulminate and lead azide. The
mercury diazoaminotetrazolate is especially very friction sensitive, but shock sensitivity was not easy to determine.
Silver azotetrazolate has been known for over 100 years, but there is not much information on it in the literature. I've made it and found it is not
as sensitive as Thiele had originally claimed, neither is the mercury salt. They don't explode by simply being contacted. The silver salt is extremely
shock sensitive, sensitivity is similar to acetone superoxide and silver fulminate. It is less sensitive to friction than the latter compound. It is a
black-orange solid when dry and it dries out in very hard clumps. Small amounts (like 5mg) could be broken up with a wooden match on tissue paper on a
hard surface (a mortar and pestle or rod are much more likely to end in explosion). It is a dangerous novelty energetic like silver fulminate. There
is a picture of it drying on a filter paper below.

I found silver azotetrazolate (AgAZT) is extremely sensitive to static shock and it detonated readily, compared to e.g. lead azide or silver
diazoaminotetrazole which didn't fire from piezoelectric sparks, even though I zapped the hell out of these two. In one of the detonations, the
voltmeter reading was about 9.2mV. Weaker discharges like 0.4mV gave no reaction. The compounds were on the edge of an aluminium strip in very small
piles or pieces then discharged. The piezoelectric sparks are strong enough to feel on the human skin, but they are not as strong as some of the
static discharges humans can generate.
When the silver azotetrazolate was put on a pretty non-conductive strip (paper) and exposed to piezoelectric jolts, the jolts despite being done
numerous times would not "seek out" the silver azotetrazolate. Even when the silver azotetrazolate was between the piezoelectric spark source and the
metal contact of the lighter (this easily causes detonation when an aluminium strip is used). The material was not conductive enough on its own to
attract the spark. The light sensitivity of silver salts which froms silver, and increases static discharge hazard.
Mechanical sparks from a lighter readily detonated silver azotetrazolate (lead azide and copper diazoaminotetrazolate did not react). AgAZT also
detonates on flame contact but reacts slower than lead azide, which was slower than silver fulminate. The loud report is significantly louder than
either of the two latter compounds. The brisance is similar to or possibly even surpasses silver azide, and so must be treated with utmost care.
Mercury azotetrazolate was a yellow gelatinous volumous precipitate which turns orange when dry. It was very friction sensitive (shock sensitivity
ought to be extreme though it wasn't tested), but a few small crystals could be gently scraped without reaction. The brisance seems similar to the
silver salt. The copper azotetrazole salt was a black-green solid when dry extremely shock and friction sensitive, significantly less brisant than the
silver salt.
Concerning Thiele's sensitivity claim of heavy metal azotetrazolates, it was quite a vague characterization and he lumped the Pb, Ag, Hg salts
together claiming they explode from slightest action. I don't know what he did but it is known shock sensitivity increases with larger amounts so
perhaps he made gram amounts and then bumped at them with a long stick when they were dry.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Formatik  | Well, another oxidant that works great for oxidizing sodium aminotetrazolate to sodium azotetrazolate is potassium persulfate (K2S2O8). I would prefer
this over the messy KMnO4, and alcohol is not needed to destroy any excess either. No filtering of the MnO2 is required either. I've tried it with
this persulfate and it worked, but I didn't characterize the yield so good because it was in the microscale, the yield is probably around the same
like by permanganate. The sodium azotetrazolate made from K2S2O8 reacted to open flame the same way as the one made from KMnO4, there was also no
reaction from hammer slamming on an iron plate. The appearance was the same also.
I also stated in the previous document ethanol can be used to wash sodium azotetrazolate, this is true as long as no preparation liquid liquor is
present, otherwise a white compound (no apparent energetic properties after isolation) begins to precipitate.
|
I am so happy someone finally reacted aminotetrazole (or at least its salt) with persulfate. Formatik, it is quite possible that you actually oxidized
it further, beyond just azotetrazole. Persulfate can oxidize nitrotetrazole to nitrotetrazole oxide. Furthermore, the azo linking group may likely
have been oxidized to an azoxy group. In other words, you may have obtained N,N’-azoxy-5,5’-bis[tetrazole-2-oxide], formula C2N10H2O3.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The thought of the persulfate potentially changing the product was a possibility I considered, but I have strong doubts about it oxidizing any further
because the sodium azotetrazolate made was very similar to the one made by permanganate, as shown by some of the mentioned basic tests (identical
appearance also).
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
But peroxysulfate can oxidize nitrotetrazole further. I see no reason why it could not similarly oxidize azotetrazole. Indeed, oxidation of
the latter should be easier.
Here is the procedure for the oxidation of nitrotetrazolate to the salt of nitrotetrazole-2-oxide,
| Quote: |
12.5g of the sodium salt of 5-nitrotetrazole is dissolved in 50mL water, then reacted with 45g of potassium peroxy-monosulfate ("Oxone") and 20g of
potassium acetate, which acts as a buffer. The solution is stirred for 24 hours at 50 degC. A solution containing 0.09 moles of tertiary amine
sulfate, such as Et3NH(+), Na(+), SO4(-2), dissolved in 200mL of water, is added. Then the 5-nitro tetrazole-2N-oxide is extracted using 300mL of
ethyl acetate. The yellow product moves into the ethyl acetate layer. The 5-nitro tetrazole-2N-oxide product may be purified by crystallization from
EtOAc or toluene, resulting in thin yellow crystals. The yield is 70%.
|
Nitrotetrazole can also be oxidized by HOF, transiently formed in solution by passing elemental fluorine into a cold liquid acrylonitrile solvent in
the presence of a lesser quantity of water.
"The Tetrazole 3-N-Oxide Synthesis" Tal Harel, Shlomo Rozen, School of Chemistry, Tel-Aviv University, Tel-Aviv, Israel. J. Org. Chem., 2010, 75
(9), pp 3141–3143
Now I am not sure if the extra oxygen is really in the "2-" or "3-" position. The inconsistency could possibly be due to the ambiguity between naming
those two positions on the tetrazole ring, because the NH group could either be in the 1- or 4- positions. But I would think that the extra oxygen
would be in the 3- position, assuming the hydrogen atom was in the 1- position (wikipedia shows this), such that the NH group could be electron
donating to the oxygen atom. Hopefully you understand what I am saying.
[Edited on 28-12-2011 by AndersHoveland]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I have made sodium azoxyazotetrazolate and this material is lighter in color than azotetrazolate, and it also reacts significantly more violently
towards flame. So I'm still in doubt a further oxidation occurred.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I was tierd when I wrote above, I meant sodium azoxytetrazolate. My descriptions on it are given in the previous pdf file on the other page of this
thread.
Anyways, Anders the reference you cited used peroxymonosulfate, whereas I used peroxydisulfate. I also may have had a reference somewhere which stated
potassium peroxydisulfate can be used in azotetrazole oxidation, but I can not find it right now. Maybe monopersulfate does from something different.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I think that the reactivity of peroxydisulfate and peroxymonosulfate are essentially the same, if not, peroxydisulfate would probably the more
powerful oxidizer. From a quick internet search, the reduction potentials apparently are,
peroxymonosulfate 1.44 V
peroxydisulfate 2.1 V
(note that these values are not for boiling solutions, and are in the absence of transition metal ions, either of which increase the oxidation
strength)
But the subject of possible differences in oxidizing strength or reactivity between the two regents are complicated, and probably deserving of a
separate discussion (a new thread).
[Edited on 29-12-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
5-Dinitromethyltetrazole
I think the most promisting energetic compound may be dinitromethyl tetrazole.
“Syntheis of 5-Dinitromethyltetrazole”, A. V. Shastin, B. L. Korsunskii, T. I. Godovikova, V. P. Lodygina. (2009) Russia
The structure of this compound has resemblance to another well-described insensitive energetic compound, diaminodinitroethylene (Fox-7).
Dinitromethyl tetrazole is somewhat acidic and can form salts.
Measurements of the explosive properties of dinitromethyl tetrazole have not been reported, but I think it would have an excellent combination of
explosive performance and stability.
The aromatic nature of the ring would stabilize the nitrogen atoms, while the two NH groups be electron-donating toward the geminal nitro
groups, stabilizing them. Many energetic tetrazole compounds have lower sensitivities than RDX, and Fox-7, which also contains the
gem-dinitro functional group, is roughly twice as resistant to impact as HMX.
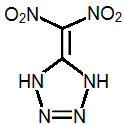
To make dinitromethyl tetrazole, one possible route might be:
React hydrazine hydrate with acetamidine to form aminoacetamidine, NH2-NH-C(=NH)-CH3, then react with acidified NaNO2 to form 5-methyl-tetrazole. Then
simply perform a mixed acid nitration. For example, one of the preparation routes of Fox-7 involves the nitration of 2-methyl imidazole.
EDIT to the above: there is a Korean paper in which it is stated that this route was attempted but failed to give the desired
product,
| Quote: |
Nitration of 5-methyltetrazole
Among the tactics for synthesizing target molecule, initial attention focused upon the nitration of 5-methyltetrazole. Previously, various starting
materials such as 2-methylimidazole, 2-methoxy-2-methyl-imidazolidine-4,5-dione,and2-methylpyrimidine-4,6-dione(4,5-dihydroxy-2-methylpyrimidine) were
nitrated and then hydrolyzed togive FOX-7 by somewhat different process. Since the methyl group was converted to dinitromethylidene moiety in all
methods, nitration of 5-methyltetrazole was attempted to afford 5-dinitromethylidene-1,4-dihydrotetrazole. But this reaction failed to proceed, and
most of the starting material was recovered.
|
Because of the aromaticity, 5-Dinitromethyltetrazole probably forms brightly colored crystals. Fox-7, for example, forms transparent yellow
bipyramidal rhomboid-shaped crystal plates.
One of the energetic salts of nitromethyltetrazole has a (calculated?) detonation velocity of 9188 m/sec @ 1.87g/cm3.
[salt 4] "Energetic mono and dibasic 5-dinitromethyltetrazolates: synthesis, properties, and particle processing", Zhuo Zeng, Haixiang Gao,
Brendan Twamley and Jean'ne M. Shreeve, J. Mater. Chem., 2007, 17, 3819-3826
[Edited on 20-1-2012 by AndersHoveland]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
http://onlinelibrary.wiley.com/doi/10.1002/prep.200900049/ab...
"Dinitromethyltetrazole and its salts: A comprehensive study"
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
One of the other, less practical, synthesis routes to dinitromethyltetrazole involved reacting trinitroacetonitrile with sodium azide and acetic acid
at (minus) -35 °C.
A. V. Shastin, T. I. Godovikova, B. L. Korsunshii, Journal of Heterocyclic Chemistry, 1998, 34, 383
(the reason for the cold temperature was because excess acetonitrile acted as the liquid solvent)
Alternatively, the sodium salt of dinitroacetonitrile can be reacted with aqueous sodium azide, but the yields from this variation are only about 10%.
For preparing the trinitroacetonitrile,
| Quote: |
Trinitroacetonitrile can be synthesized by the nitration of cyanoacetic acid with a solution of sulfur dioxide and 98+% concentrated nitric acid in
carbon tetrachloride, with 73-77% yields. The trinitroacetonitrile can be stored as a solution in the carbon tetrachloride, and need not be isolated
for further use on other reactions.
NCCH2C(=O)OH + (3) HNO3 + (3)SO2 -- > NCC(NO2)3 + CO2 + (3) H2SO4
Trinitroacetonitrile is a colorless, camphor-like, crystalline compound melting at 41.5 °, and detonating violently at 220°. It hydrolyzes to carbon
dioxide and the ammonium salt of nitroform by water or alcohol at ordinary temperatures.
|
There is another synthesis route that uses Fox-7 as the precursor to make dinitromethyltetrazole, which has already been discussed on this forum.
Here is a description of the first published synthesis of the compound, where the researchers referred to 5-dinitromethyltetrazole as "N2FOX-7":
| Quote: |
...we found a more convenient method to get [ethyl 5-tetrazolyldinitro-acetate] ETDNA. Because of acidity on the methylene position of
[ethyltetrazolylacetate] ETA, which possesses both carbonyl group and tetrazole moiety, we considered it's possible to introduce nitro group directly.
Thus we carried out the reaction of ethyl 5-tetrazolylacetate with mixed acids and obtained ETDNA in high yield, resulting that two nitro groups were
successfully introduced in one pot reaction.
Synthesis of TDNM and it’s salts
With the necessary intermediates in hand, we sought to construct the N2FOX-7 using decarboxylation and elimination. Thus, various conditions were
tried. When ethyl 5-tetrazolyldinitro-acetate was treated with water, 5-dinitromethyltetrazole was readily given. Hydrolysis followed by
decarboxylation took place completely within 2 h at 50 °C. In the treatment of 5-dinitromethyltetrazole with KOH, dipotassium salt and monopotassium
were obtained even in lower temperature, depending upon the equivalent of KOH. Mono- and di-potassium salt were confirmed by Inductively coupled
plasma (ICP) mass analysis. Meanwhile, only mono ammonium salt was given in the reaction with ammonia regardless of equivalents.
5-Dinitromethyltetrazole was also afforded by an acid treatment of the salts. As a result, hydrolysis and decarboxylation of ETDNA was achieved not
only underacidic or basic condition but under aqueous one at room temperature.This process would be much more efficient and safer to obtain TDMN and
its salts than the previous methods. Preliminary experiments showed that these compounds have some explosive properties.
ethyltetrazolylacetate (ETA)
N4HC-CH2-C(=O)-O-CH2CH3
ethyl 5-tetrazolyldinitro-acetate (ETDNA)
N4HC-C(NO2)2-C(=O)-O-CH2CH3
|
Synthesis and Characterization of High EnergeticTetrazole and Furoxan Derivatives, (Korea)
[Edited on 21-1-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
To make an additional comment,
Quote: Originally posted by AndersHoveland  |
Nitration of 5-methyltetrazole
various starting materials such as 2-methylimidazole,
2-methoxy-2-methyl-imidazolidine-4,5-dione,and2-methylpyrimidine-4,6-dione(4,5-dihydroxy-2-methylpyrimidine) were nitrated and then hydrolyzed to give
FOX-7 by somewhat different process. Since the methyl group was converted to dinitromethylidene moiety in all methods, nitration of 5-methyltetrazole
was attempted to afford 5-dinitromethylidene-1,4-dihydrotetrazole. But this reaction failed to proceed, and most of the starting material was
recovered.
|
The reason that the nitration of 5-methyltetrazole failed to proceed is probably the same reason that nitration of plain 1,2,3-triazole is essentially
impossible. I suspect that the electron-withdrawing tetrazole ring pulls away electric charge from the methyl group, giving it a partial positive
charge and effectively shielding it from interaction with nitronium ions, which is the mechanism of reaction in most nitrations.
To bypass this difficulty, one idea would be to brominate the methyl group with bromine in the presence of uv light. Carbon tetrachloride would be a
good choice of solvent for this radical-intitiated reaction. The bromomethyltetrazole could then be reacted with sodium nitrite in DMSO to substitute
the bromine atom for a nitro group, to obtain nitromethyltetrazole. This could then be nitrated to give the final dinitromethyltetrazole product.
4-nitro-1,2,3-triazole, for example, can be readily nitrated to 4,5-dinitro-1,2,3-triazole.
For more details these types of substitution reactions, included is the procedure for nitroethane from ethyl bromide and sodium nitrite,
| Quote: |
from ethyl bromide (iodide) and sodium nitrite (dmf)
32.5 grams of ethyl bromide (0.3 moles) was poured into a stirred solution of 600ml dimethylformamide and 36 grams dry NaNO2 (0.52 mole) in a beaker
standing in a water bath keeping the solution at room temperature as the reaction is slightly exothermic. Always keep the solution out of direct
sunlight. The stirring was continued for six hours. After that, the reaction mixture was poured into a 2500 ml beaker or flask, containing 1500 ml
ice-water and 100 ml of petroleum ether. The petroleum ether layer was poured off and saved, and the aqueous phase was extracted four more times
with 100 ml of petroleum ether each, where after the organic extracts were pooled, and in turn was washed with 4x75ml of water. The remaining
organic phase was dried over magnesium sulfate, filtered, and the petroleum ether was removed by distillation under reduced pressure on a water bath,
which temperature was allowed to slowly rise to about 65°C. The residue, consisting of crude nitroethane was distilled under ordinary pressure
(preferably with a small distillation column) to give 60% of product, boiling at 114-116°C.
The ethyl bromide reacts with NaNO2, forming nitroethane and ethyl nitrite.
This method can be varied in a few ways. Firstly, dimethyl sulfoxide (DMSO) can be substituted for the dimethylformamide (DMF) as solvent.
Ethylene glycol also works as solvent, but the reaction proceeds pretty sluggishly in this medium, allowing for side reactions, such as this: RH-NO2 +
R-ONO => R-(NO)NO2 + R-OH. KNO2 can also be used instead of NaNO2. If NaNO2 is used in DMF, 30g (0.5 mol) of urea can also be added as nitrite
scavenger to minimize side reactions, as well as simultaneously increasing the solubility of the NaNO2 and thereby significantly speeding up the
reaction.
If the ethyl bromide is substituted with ethyl iodide, the required reaction time is decreased to only 2.5 hours instead of 6 hours. In case ethyl
iodide is employed, a slight change in the above procedure needs to be done. The pooled pet ether extracts should be washed with 2x75ml 10% sodium
thiosulfate, followed by 2x75ml water, instead of 4x75ml water as above. This to remove small amounts of free iodine
|
|
|
|
NatashaJurievna
Harmless

Posts: 9
Registered: 5-2-2012
Location: Carpathians
Member Is Offline
Mood: Too warlike, not friendly
|
|
Back to 5-AT preparation
I'm surprised that no one mentions the Czechoslovakian patent no. 190055
http://spisy.upv.cz/Patents/FirstPages/FPPV0190/0190055.pdf

Translation of the text above:
164 grams of aminoguanidine sulfate were dissolved in 250 ml of hot 2,5N HCl. After the sulfate dissolved, the solution was cooled to 20 °C and 72
grams of NaNO2 in 100 ml of water were added dropwise at a temperature no more than 40 °C. When the nitrite solution was added, mixing was continued
for 1 hour. Then 100 grams of sodium acetate were added, solution heated to boil and boiled for 20 minutes. After cooling, white crystals were
filtered off and washed with a small amount of cold water.
Melting point 199 - 200 °C. Yield 69 g or 83% of the theoretical yield.

[Edited on 5-2-2012 by NatashaJurievna]
|
|
|
mabuse_
Hazard to Self
 
Posts: 56
Registered: 3-6-2010
Member Is Offline
Mood: No Mood
|
|
I just managed to get the starting materials for 5ATZ together and got my first batch ready.
http://www.wydawnictwa.ipo.waw.pl/cejem/2-2010/full/Sabate.p...
Has anyone here ever tried to reproduce the ethylenediamine compounds 3 and 4?
They seem to have the most desirable characteristics, ESD insensitive, extremely insensitive against shock & friction but apparently very
sensitive to thermal shock.
To good to be true...
|
|
|
NatashaJurievna
Harmless

Posts: 9
Registered: 5-2-2012
Location: Carpathians
Member Is Offline
Mood: Too warlike, not friendly
|
|
Quote: Originally posted by mabuse_  | Has anyone here ever tried to reproduce the ethylenediamine compounds 3 and 4?
They seem to have the most desirable characteristics, ESD insensitive, extremely insensitive against shock & friction but apparently very
sensitive to thermal shock.
To good to be true... |
They seem to be interesting but I don't like somewhat the highly negative oxygen balance of the compounds. From the insensitive primary explosives
based on tetrazole derivates I prefer the disilver aminotetrazole nitrate (double salt of silver nitrate and silver aminotetrazolate) though not cheap
because of silver (good old 90's...).
|
|
|
mabuse_
Hazard to Self
 
Posts: 56
Registered: 3-6-2010
Member Is Offline
Mood: No Mood
|
|
That's true.
To bad they did not deliver any performance figures.
| Quote: |
double salt of silver nitrate and silver aminotetrazolate |
Do you have any data on that?
I find the price for the silver not that important, you don't want and don't need much anyway. Considering the rest of the raw materials the silver is
a drop in the bucket
|
|
|
NatashaJurievna
Harmless

Posts: 9
Registered: 5-2-2012
Location: Carpathians
Member Is Offline
Mood: Too warlike, not friendly
|
|
Quote: Originally posted by mabuse_  | That's true.
To bad they did not deliver any performance figures.
| Quote: |
double salt of silver nitrate and silver aminotetrazolate |
Do you have any data on that?
I find the price for the silver not that important, you don't want and don't need much anyway. Considering the rest of the raw materials the silver is
a drop in the bucket |
Where the nitrate is mentioned:
5-Aminotetrazoles and Silver-based Primary Explosives
While the nitrate is not as friction insensitive as the ethylenediamine nitrotetrazolate complexes and is sensitive to ESD, working with it is pretty
safe in comparison with lead azide. I've not yet tested its initiating properties but seems to be a strong primary explosive. And yes, the silver is
the more accessible part of the compound 
Is there any practical reason to nitration of ATZ, while it makes decent primary explosives? Ok, ok, I know. People are curious. I wonder if there are
possible any usable ammonia/5-ATZ/Co(III) perchlorate or nitrite complexes.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Nitration of 5-methyltetrazole
Quote: Originally posted by AndersHoveland  | "...nitration of 5-methyltetrazole was attempted to afford 5-dinitromethylidene-1,4-dihydrotetrazole. But this reaction failed to proceed, and most
of the starting material was recovered."
The reason that the nitration of 5-methyltetrazole failed to proceed is probably the same reason that nitration of plain 1,2,3-triazole is essentially
impossible. I suspect that the electron-withdrawing tetrazole ring pulls away electric charge from the methyl group, giving it a partial positive
charge and effectively shielding it from interaction with nitronium ions, which is the mechanism of reaction in most nitrations.
|
There is another route that could potentially be used to nitrate 5-methyltetrazole, by using a small quantity of N-hydroxyphthalimide as a catalyst:
| Quote: |
Catalytic nitration of alkanes with nitric acid was first successfully achieved by the use of N-hydroxyphthalimide (NHPI) under mild conditions; the
key to the present nitration was found to be the in situ generation of NO2 and phthalimide N-oxyl radical by the reaction of NHPI with nitric acid.

http://pubs.rsc.org/en/content/articlelanding/2001/cc/b10237...
"Nitration of alkanes with nitric acid catalyzed by N-hydroxyphthalimide", Shinji Isozaki , Yoshiki Nishiwaki , Satoshi Sakaguchi and Yasutaka
Ishii, Chem. Commun., 2001
|
The formation of intermediate radicals would bypass the charge shielding effect on the methyl group. As soon as the first nitro group is added, the
molecule should then easily be able to undergo the normal nitration mechanism to add a second nitro group.
| Quote: |
nitromethyltetrazole could then be nitrated to give the final dinitromethyltetrazole product. 4-nitro-1,2,3-triazole, for example, can be readily
nitrated to 4,5-dinitro-1,2,3-triazole.
|
If this works, an industrial process to this explosive compound could be fairly straightforward, using only acetamidine, hydrazine, nitrous acid
[generated in reaction], nitric acid, and using NHPI as a catalyst.
see the diagram below:
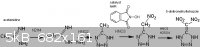
For the condensation of acetamidine with hydrazine (which displaces off ammonia), "Preparation of hydrazidines" US 4443629
This has already been extensively discussed elsewhere in this forum, but nitrous acid (from NaNO2 + HCl in solution) oxidizes aminoguanidine salts
into guanyl azide N=N=N-C(=NH)NH2, which cyclizes into aminotetrazole (HN4C)NH2 when boiled under alkaline conditions. This takes several hours and
gives 70-85% yield.
[Edited on 24-2-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Just compare the structure of 5-dinitromethyltetrazole (DNMT) to Fox-7, which is both powerful (more than RDX), and has a lower sensitivity. DNMT has
two less hydrogen atoms (meaning better oxygen balance), and two more nitrogen atoms. The structure of DNMT is related to Fox-7, despite the fact that
DNMT is also a tetrazole.
I do not know how sensitive DNMT is, but it is quite possible that DNMT may have a high enough resistance to impact to be used as a
commercial/military explosive.
Fox-7 is about twice as resistant to impact as RDX. And Fox-7 has a detonation velocity of 8870 m/sec.
The nitrate salt of 5-aminotetrazole, for example, is both more powerful and less sensitive than RDX.
The detonation velocity for 5-nitrotetrazole is above 8.5 km/sec, and its sensitivity is 25cm.
V.A.Ostrovskii, G.I.Koldobskii, Russian Chemical Journal, 41, 84, (1997).
(for comparison, HMX is slightly more sensitive, with a drop height of 23cm) Most of the salts of 5-nitrotetrazole, however, are much more sensitive.
So looking at the properties of Fox-7 and some of the tetrazoles, it seems very probable that the sensitivity of DNMT is not too high. DNMT should be
a very powerful explosive, the detonation velocity probably approaches 10 km/sec. Considering the predicted excellent performance, and low sensitivity
DNMT would seem to hold much potential for practical application.
[Edited on 25-2-2012 by AndersHoveland]
|
|
|
woelen
|
Thread Pruned
13-3-2012 at 05:40 |
fenolazul
Harmless

Posts: 4
Registered: 28-6-2012
Member Is Offline
Mood: No Mood
|
|
Synthesis of acid copper 5-Nitrotetrazolate (CuNT)
Quote: Originally posted by Engager  | Synthesis of acid copper 5-Nitrotetrazolate (CuNT) from 5-aminotetrazole
Prepare solution 20.8g sodium nitrite and 11g of copper sulphate (CuSO4*5H2O) in 60 ml of hot water, resulting
solution contains copper nitrite and has dark green color. Prepare solution of 10.3g aminotetrazole (ATZ) and 0.4g CuSO4*5H2O in 12.8 ml 70% nitric
acid + 120 ml of water. Diazotetrazole is intermediate in nitrotetrazole synthesis, it can explode in solution if concentration will reach 2% from the
slightless stimulus, even at 0C. Microexplosions are not dangerous but acting on nerves. To completely avoid them, slow addition, effective stiring
and carefull temperature control are essential. Small adition of copper sulphate to ATZ solution is essential to avoid microexplosions in drops, on
contact with nitrogen oxides, escaping from reaction mixture. Copper nitrite solution is placed on ice bath and cooled to 5C, then solution of ATZ in
nitric acid, is added slowly with stirring (perfectly drop by drop). During addition temperature of reaction mixture must be kept below 15C all times.
If mixture begins to foam, addition is paused and mixture is well stirred until foam dissapears before next portions of ATZ solution are added
(foaming is result of HNO2 decomposition to water and NOx if it's concentration is too high). Reaction is proceeding smoothly, without
microexplosions, with small evolution of NOx, if conditions are carefully controlled. Whole addition process takes time about 1.5 hours. Close to end
of addition mixture becomes thicker, and changes color to green- blue (similar with homemade CuCO3*Cu(OH)2). After addition is completed mixture is
left to sit in ice bath for 15 minutes, after that 14 ml of 70% nitric acid + 6 ml of water is added with stirring. Reaction mixture is left for 1
hour, solid precipitate of acid copper salt of nitrotetrazole is filtered off, washed with 5.72 ml HNO3 + 44 ml H2O and with three portions of 50 ml
H2O. Yield is about 85%. Product is bluish - green crystals, almost insoluble in cold water.
Warning!!! Acid copper salt of nitrotetrazole is powerfull and sensitive explosive.
It is almost completely safe then wet, but in dry state it can explode violently on friction, impact or heating. Safety precautions must be
remembered all times.
Below are the photos of process. Left photo shows solutions of copper nitrite (left one) and ATZ in nitric acid with
CuSO4 added (right one). Photo in the middle shows ice bath with sitting reaction flask, and termocouple temperature control. Right photo shows
reaction product on filter.
Reaction sheme:
Acid copper salt of nitrotetrazole can be easily converted to soluble salts of nitrotetrazole by heating in solution
of corresponding hydroxides. For example solution of sodium 5-nitrotetrazolate can be prepared by boiling acid copper salt in NaOH solution:
Cu(NT)2*HNT + 3NaOH => 3NaNT + CuO+ 2H2O. Solid black copper oxide is removed by filtering, pure solution of NaNT can be concentrated to separate
solid salt, or can be used dirrectly for further reactions.
[Edited on 14-9-2007 by Engager] |
Quote: Originally posted by Engager  | Synthesis of acid copper 5-Nitrotetrazolate (CuNT) from 5-aminotetrazole
Prepare solution 20.8g sodium nitrite and 11g of copper sulphate (CuSO4*5H2O) in 60 ml of hot water, resulting
solution contains copper nitrite and has dark green color. Prepare solution of 10.3g aminotetrazole (ATZ) and 0.4g CuSO4*5H2O in 12.8 ml 70% nitric
acid + 120 ml of water. Diazotetrazole is intermediate in nitrotetrazole synthesis, it can explode in solution if concentration will reach 2% from the
slightless stimulus, even at 0C. Microexplosions are not dangerous but acting on nerves. To completely avoid them, slow addition, effective stiring
and carefull temperature control are essential. Small adition of copper sulphate to ATZ solution is essential to avoid microexplosions in drops, on
contact with nitrogen oxides, escaping from reaction mixture. Copper nitrite solution is placed on ice bath and cooled to 5C, then solution of ATZ in
nitric acid, is added slowly with stirring (perfectly drop by drop). During addition temperature of reaction mixture must be kept below 15C all times.
If mixture begins to foam, addition is paused and mixture is well stirred until foam dissapears before next portions of ATZ solution are added
(foaming is result of HNO2 decomposition to water and NOx if it's concentration is too high). Reaction is proceeding smoothly, without
microexplosions, with small evolution of NOx, if conditions are carefully controlled. Whole addition process takes time about 1.5 hours. Close to end
of addition mixture becomes thicker, and changes color to green- blue (similar with homemade CuCO3*Cu(OH)2). After addition is completed mixture is
left to sit in ice bath for 15 minutes, after that 14 ml of 70% nitric acid + 6 ml of water is added with stirring. Reaction mixture is left for 1
hour, solid precipitate of acid copper salt of nitrotetrazole is filtered off, washed with 5.72 ml HNO3 + 44 ml H2O and with three portions of 50 ml
H2O. Yield is about 85%. Product is bluish - green crystals, almost insoluble in cold water.
Warning!!! Acid copper salt of nitrotetrazole is powerfull and sensitive explosive.
It is almost completely safe then wet, but in dry state it can explode violently on friction, impact or heating. Safety precautions must be
remembered all times.
Below are the photos of process. Left photo shows solutions of copper nitrite (left one) and ATZ in nitric acid with
CuSO4 added (right one). Photo in the middle shows ice bath with sitting reaction flask, and termocouple temperature control. Right photo shows
reaction product on filter.
Reaction sheme:
Acid copper salt of nitrotetrazole can be easily converted to soluble salts of nitrotetrazole by heating in solution
of corresponding hydroxides. For example solution of sodium 5-nitrotetrazolate can be prepared by boiling acid copper salt in NaOH solution:
Cu(NT)2*HNT + 3NaOH => 3NaNT + CuO+ 2H2O. Solid black copper oxide is removed by filtering, pure solution of NaNT can be concentrated to separate
solid salt, or can be used dirrectly for further reactions.
[Edited on 14-9-2007 by Engager] |
Best regards from the hell.
I finally managed to synthesize this "magical" product: acid copper 5-Nitrotetrazolate (CuNT) from 5-AMINO-1H-TETRAZOLE MONOHIDRATO.
CuNT it is powerful and “green booster” primary explosive, following the valuable -step by step- instructions by Engager. Many thanks, master
chemical!
But I have several questions, friends:
1º Once synthesized acid copper 5-Nitrotetrazolate, if it is stable for long periods of time. Years, even...?
2º Can be stored dry or always for safety must always be kept moist, immersed in distilled water and drying the exact amount to be used?
3º In the dry state is sensitive to hammer percussion?
(I have severely beaten the product between an anvil and a hammer and there is no outbreak)
4º Is sensitive to friction?
5º Is sensitive to static electricity?
6º Their contact is compatible with plastics materials, metals or metalloids without forming salts unstable or in contact with aluminum, steel,
iron, antimony, etc. ... ?
7º Can be overpressed?
Finally and honestly, Engager/Sciencemadness friends, for personal experience: this powerfull compound is really stable and safe to handle?
I wish all the information possible about acid copper 5-Nitrotetrazolate , please.
Primary explosives handling, errors do not give second chances. 
[Edited on 30-6-2012 by fenolazul]
[Edited on 30-6-2012 by fenolazul]

[Edited on 30-6-2012 by fenolazul]

[Edited on 30-6-2012 by fenolazul]
[Edited on 30-6-2012 by fenolazul]
|
|
|
fenolazul
Harmless

Posts: 4
Registered: 28-6-2012
Member Is Offline
Mood: No Mood
|
|
Any information to acid copper 5-Nitrotetrazolate (CuNT) from 5-AMINO-1H-TETRAZOLE MONOHIDRATO?
No expert has nothing to say? 
[Edited on 4-7-2012 by fenolazul]
[Edited on 4-7-2012 by fenolazul]
|
|
|
| Pages:
1
..
10
11
12
13
14
..
23 |
|