| Pages:
1
2 |
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
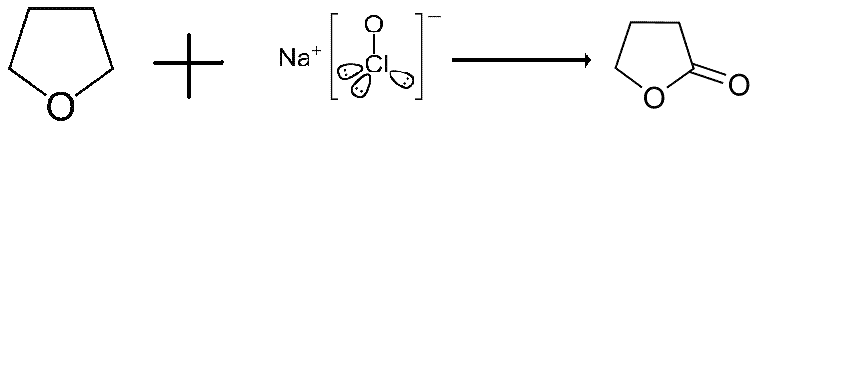
Oxidising THF using calcium hypochlorite
I was wondering if anyone who has performed this oxidation can let me know the importance of vaccumm filtration in the whole scheme?
Is it workable without it?
Or will shit hit the fan?
Direct conversion of ethers to esters by trichloroisocyanuric acid
Juenge and Beal
Tetrahedron Letters vol.9, No. 55, pp 5819-5820 (1968)
http://www.sciencemadness.org/talk/viewthread.php?action=att...
Is the article this post is based loosely around. I don't have a copy of the article. However, i have requested it and will update this post
accordingly.
Thanks in advance and thanks to solo for the article
[Edited on 17-3-2007 by gambler]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Never done it.
Oxidizing THF seems like a good way to make peroxides and blow your head off!
It's a 6 membered ring and is not likely to change (ring strain is actually 0 kJ/mol). Of interest is taht they have managed to polymerize THF via
ring-opening-polymerization via cationic means (very cold).
Since your still here, I am interested in hearing how the rxn went!
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
They probably want to remove fine particles of calcium salts by vacuum filtration.
If you are concerned about the cost of a mechanical pump, consider a water aspirator pump (Nalgene) instead.
Or a Nalgene hand pump. Both are inexpensive. And more than adequate for such a filtration. You will need a filtering flask, an appropriate funnel
(preferably fritted disk type Buchner) with a rubber adapter between the two and a length of vacuum hose (usually red gum rubber, thick walled).
@O3, I think our friend here wants to open the THF up to BDO/GBA with a view to closing it back up as GBL. That is the sole driving force behind such
questions and it has more to do with blowing their little heads than it does with peroxides.
[Edited on 17-3-2007 by Sauron]
|
|
|
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
Thankyou for the advice Sauron.
I will invest in a water aspirator as the fine particles do concern me.
Thanks for the heads up.
Apart from that it seems like a relatively simply procedure much more so than the conversion of GABA. But time will tell
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
the last person here who talked about making ether peroxides was called zepplin by his handle and now Gambler, appropriate names for people who want
to play with ether peroxides.
not to sound condescending it's just a bad idea to mix an oxidizer like calcium hypochlorite with thf.
anytime you do that an explosion will likely ensue.
[Edited on 17-3-2007 by jon]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Errm, vacuum filtration is usually done to speed things up. In fact, it's usually inferior to normal gravity filtration because you'll suck very fin
grit through the filter.
Also, hypochlorite can not introduce peroxides. You need oxygen and UV or hydrogen peroxide for that. Pretty simple to avoid: avoid sunlight exposure.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
Just a few questions regarding this reaction..
- What is the other compound produced by the oxidation of the THF
- Forgive me for asking a foolish question – nonetheless I will proceed. What dangers are present in this reaction (the oxidation is run between
–10c and 10c).
- If extreme dangers are present then I won’t proceed. However, I have seen successful reports of this reaction and if proper care is taken im sure
danger can be minimised.
In anticipation of your well articulated responses.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Well, first some errata (while I'm not dead tired):
THF is 5 membered, not six. The steric energy of the ring is around 5kJ/mol-but, kinetically the 5 membered ring is favored. The result? A very stable
ring (Odian, 2nd Ed.). THF has been polymerized, pyran (0 kJ/mol) has not.
The NaOCl may not make peroxides, but unless the solvents are degassed and thoroughly protected from air, the radicals you will get will be quenched
by O2 to yield O'.
Regardless, I am interested in whether or not this thing works.
A method that probably will work (for scientific purposes only) involves the oxidation with RuO4 of ethers to yield the corresponding ester. See
Monson for this. RuO2 is expensive, but can be recycled.
Alternatively, the ketone can be oxidized to the ester using either perbenzoic acid or K-persulfate(also in Monson).
Anyway, be careful (and, chlorination side products can occur, which would be be too good to ingest, but that's not what you want to do, right?).
Yup,
O3
[Edited on 20-3-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
dapper
Hazard to Self
 
Posts: 66
Registered: 8-11-2006
Member Is Offline
Mood: No Mood
|
|
Say the hypochlorite oxidation works out, how is the steam distillation of the product? Any comments?
|
|
|
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
This is the text that I derived the idea from (full thread available at WD).
I will quote below the authors instructions.
Whilst it seems like a simple procedure keeping it below 10 degrees seems to be the key and the use of vaccumm filtration.
Discussion in regards to feasibility (from a technical not an economic standpoint) would be appreciated.
Thankyou again
“Mix 5 grams of THF, 100ml denatured alcohol (hardware store stuff), 5ml 31%
HCL (hardware store stuff) in a 250ml conical flask. Put the flask in an
ice bath and allow the temperature to fall to near 0C. It is important that
the reaction not rise above 10C during the entire process.
Under constant stirring add 10g of Ca-hypochlorite (pool supply stuff) a
gram at a time monitoring the temperature often to be sure it doesn't rise
above the 10C point. This is actually easy to do, the reaction proceeds
slowly by adding the solid to the liquid in the absence of water and the
temperature is easy to control as long as the additions are done carefully.
This is important, add the solid to the cooled liquid.
Let the reagents react for 30 minutes in the ice bath, then remove the ice
bath. Monitor the temperature for another minute to be sure it doesn't
spontaneously begin to rise rapidly. Once the solution has stabilized at
room temperature begin slowly heating the solution. The solution will have
a largish amount of powdery solids in it. When the alcohol has just begun
to boil, vacuum filter it through the finest filter paper you have.
Vacuum filtering is a requirement. The solution has become viscous and the
fine solids will plug up any gravity based filter. The filtered solution
will still have some of the fine solids in it, but this is not a problem.
Just get as much out as you can by careful filtering. Rinse the solids with
some more denatured alcohol to assure the newly created GBL is carried
through the filter.
Isolating the GBL at this point is hard, so just proceed to making GHB which
will be easier to separate and is actually the goal of this process anyway.
Add 4g of NaOH to the solution and start stirring. After a few minutes
you'll notice that as the NaOH dissolves another fine white solid is formed.
Let the reaction progress for about 30 minutes checking every few minutes to
make sure the solution doesn't start to solidify. If it does solidify, just
add more alcohol until stirring is easy again. Once again, heat the
solution and vacuum filter it. Remove as much of the solids as you can
while filtering and be sure to use vacuum filtering, gravity filtering will
take all day. Be sure to rinse the filter and the solids with alcohol to
get all the dissolved GHB.
Save the solids from this process, you will be comparing them later in the
process. Just scrape them off the filter and set aside to dry.
Evaporate the alcohol and the small amount of water that has been created
and absorbed from the air by heating GENTLY for a couple of hours.
Alternately distill most of the alcohol off and evaporate the rest. When
completely dry this will leave a residue of beige GHB, some salt and some
CaCl. First scrape the crunchy powder up from the evaporation dish and put
in a flask and boil with acetone. Allow the acetone to cool to room
temperature and filter. Retain the solids and discard the acetone. This
will get rid of any remaining unreacted solvents that have been hiding in
the crystal matrix. Dry the acetone wet solids. Now dissolve the solid in
fresh alcohol (dry the alcohol through anhydrous magnesium sulfate). Not
all of the solids will dissolve, the salt and CaCl will remain solid and can
be filtered out. Evaporating the Alcohol will give you nice clean GHB
powder that must be tightly sealed up while still hot to prevent it from
gathering water from the surrounding air.
Now look at the solids you saved from the first filtration above. If they
are dry and haven't started collecting water from the surrounding air, just
discard them. If they have started to get wet by absorbing water from the
surrounding air, put them through the purification process and recover any
GHB that was trapped in there.
Just to be sure you didn't add too much NaOH and created a nasty lye mix
with the GHB, dissolve a small amount of the new GHB in water and check the
Ph. If it isn't neutral or slightly acidic, neutralize and clean up the
powder again. This hasn't been necessary in any of my experiment
|
|
|
mono
Harmless

Posts: 10
Registered: 16-3-2007
Member Is Offline
Mood: No Mood
|
|
are you sure you have the right reference? (not able to axx the link).
at rhodium's page its:
Ref. 1 5554344; Journal; Nwaukwa, Stephen O.; Keehn, Philip M.; TELEAY; Tetrahedron Lett.; EN; 23; 1982; 35-38;
anyway the product isn't really easy to steam distill. (this was the case after 1,4bdo dehydrogenation)
Liberty
1a. The condition of being free from restriction or control.
b. The right and power to act, believe, or express oneself in a manner of one\'s own choosing.
c. The condition of being physically and legally free from confinement, servitude, or forced labor.
|
|
|
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
Haha
Havent had much of a chance to look in this week.
Mono: You are absolutely right i have not acquired the correct reference and will seek to rectify that this weekend. I wasn't planning on steam
distilling? Are you quite sure that is necesary?
Thanks
|
|
|
menthol
Harmless

Posts: 4
Registered: 9-4-2007
Member Is Offline
Mood: No Mood
|
|
What's the expected yield from this?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Don't go drinking this shit on top of booze and go into a coma! This shit is just like getting drunk. Anyway, if you insist, (and who am I to talk?)
at least have the class to produce a solid crystalline product!
|
|
|
menthol
Harmless

Posts: 4
Registered: 9-4-2007
Member Is Offline
Mood: No Mood
|
|
The more I read this synth, the easier it seems. That's a far cry from actually doing it, of course.
Where is it most likely to fail? Assuming the oxidation happens in the absence of UV and is properly cooled, peroxides seem unlikely. What else can
go wrong with oxidation?
How would a kitchen chemist know the quality of the finished product, or even that it was the right finished product? Reduction to a crystalline
product would obviously be desirable, but then what? Before a bioassay, what steps would you take to ensure you weren't about to dose yourself with
something lethal?
|
|
|
gambler
Harmless

Posts: 44
Registered: 30-6-2006
Member Is Offline
Mood: No Mood
|
|
In terms of explosions it was my understanding that most THF that is commercially available is inhibited with BHT or other inhibitor to prevent the
formation of explosive peroxides.
Uninhibited THF is typically sold specifically as a high purity reagent for sensitive chromatographic separations / purifications where the inhibitor
may interfere with the separation, detection, purification, sample etc. IIRC.
That being said check your MSDS. However, most will find it inhibited.
| Quote: | Originally posted by Ozone
Never done it.
Oxidizing THF seems like a good way to make peroxides and blow your head off!
It's a 6 membered ring and is not likely to change (ring strain is actually 0 kJ/mol). Of interest is taht they have managed to polymerize THF via
ring-opening-polymerization via cationic means (very cold).
Since your still here, I am interested in hearing how the rxn went!
Cheers,
O3 |
|
|
|
menthol
Harmless

Posts: 4
Registered: 9-4-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by gambler
In terms of explosions it was my understanding that most THF that is commercially available is inhibited with BHT or other inhibitor to prevent the
formation of explosive peroxides.
|
What does that do to the final product of this oxidation?
On the subject of peroxides, would their production be reduced/nullified with the use of an even colder bath, say with the use of dry ice?
If peroxides are formed, would they show up in the final product, or could they be evaporated off over very low heat during the GHB extraction phase
or pulled out of the finished product with the acetone in the final boil?
(Incidentally, the concept of boiling acetone worries me more than the peroxides...)
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Acetone will boil in a pot full of hot water with no flame anywhere near it. I used this "technique" to reduce the solution volume and recover more
ASA when I was purifying some aspirin. I've had a beaker of it catch fire before, which is not fun when it's boiling. *fireball* (watch your eyebrows)
Forunately, it was in an appropriate place with nothing flammable near it and I quickly put it out by inverting a bowl over the beaker. As long as
you have some ventilation, the boiling acetone is not an issue. I'd be significantly more worried about peroxides blowing up on me.
I feel like I've read this thread before. It sounds very familiar...maybe that was the old one by zepplin. Don't kill yourself, at any rate.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
menthol
Harmless

Posts: 4
Registered: 9-4-2007
Member Is Offline
Mood: No Mood
|
|
Another crazy question:
If I can't find a supplier of THF, or I deem buying ACS THF too risky, I found an idea from a post a year ago to refine THF from PVC cement:
[Quote]This is what you do, i just tryed this 2 minutes before typing this so it works. Take the pipe cement and dump it in a beaker water with about
3/4 the volume of the cement. Then take a stir rod and mix well, the resin with form a ball around ur stick, keep mixing until ur sure the water has
disolved all of the THF and the resin is no longer liquidy but a soilid ball. Now filter the resin from the mix, and squeze even drop out of the resin
ball. Dump in a bit more than enough salt to saturate the water and poof organic layer floats to the top, use a sep funnel or just a plastic bag to
seperate the two layers. Theres your THF and any other solvents in the can. Thats as far as i got. Now to get rid of the rest add this to some water
and add in some sulfite to kill the peroxides and get rid of the ketones and seperate again. Then you have your THF.[/Quote]
I thought THF was moderately soluble in water, so would it still separate from the crap+water layer? Is that what the salt's for?
When he says 'sulfite', does he mean any sulfite? With sodium sulfite work fine?
|
|
|
slim
Harmless

Posts: 2
Registered: 21-10-2007
Member Is Offline
Mood: No Mood
|
|
(most important to least important; everything is important)
>CaCl
Is this Calcium chloride or CaCl2 ?
> (dry the alcohol through anhydrous magnesium sulfate)
through??? I have read about adding anhydrous magnesium sulfate. How much or how to know when enough has been added? Like other chemical drying, is
it important to filter after this step prior to evaporating with heat?
>Dry the acetone wet solids.
Is there something more specific; use some heat?
After looking at the data-sheet, I believe that small amounts of this stuff is not very very toxic. I'm more concerned about the CaCl (assuming this
is CaCl2).
>full thread available at WD
Do you have a link?
>boiling acetone
that will not scale (vapors); not in my house .. a condenser would do the trick until a good substitute is found
|
|
|
slim
Harmless

Posts: 2
Registered: 21-10-2007
Member Is Offline
Mood: No Mood
|
|
>Mix 5 grams of THF
Is this 5 grams of liquid THF? Otherwise how many milliliters should be used?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ask yourself "what is the density of THF at ambient temperature in g/ml?"
Then do the arithmetic.
If you haven't got a Merck or CRC Handbook handy, go to www.acros.be and in the Catalog Search box at left type tetrahydrofuran and hit GO. The density will be displayed.
Sic gorgeamus a los subjectatus nunc.
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
I thought THF was moderately soluble in water, so would it still separate from the crap+water layer? Is that what the
salt's for?
No it doesn't separate - at least not when i added PVC cement to water and then salted it - the pvc does ball up though - remove it before you salt
(was trying to get some THF - its a nice solvent)
As for using GBL - its dangerous - why risk it - if you want a downer get some benzo's from your Doctor.. fake some anxiety disorder - at least you
won't die from those (unlikely) - unless you have another use in mind..
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
You can tell when the solvent is dry when you have "snowy" particles of sulfate floating around in it. The first portion added will clump up and
settle to the bottom quickly. Also, I thought GHB was one of those drugs that "wasn't that bad" as long as you didn't drink with it - in which case
you would end up in hospital.
Also.... now I know why when I went to a chem supplier a few years back he said, jokingly but seriously, just don't ask me for any 4 substituted
butyric acids. I didn't get it at the time 
|
|
|
avarage
Harmless

Posts: 1
Registered: 29-2-2008
Member Is Offline
Mood: No Mood
|
|
by the description of the experiment am i right to say that in order to convert half a drum of THF you would need 90kg of CaCl2O2, 100 L of methanol,
and 50L of HCl, give or take??? thats alot!
|
|
|
| Pages:
1
2 |