| Pages:
1
2 |
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Ammonia production from ammonium sulfate & sodium base
From what I have seen, one of the most commonly available compounds to make ammonia is ammonium sulfate. I've tried urea as well and while easy to
produce gas initially, it seems to only produce for a short time then produces a mix of NH3 and other vaporized compounds that clog the glass tubing
very quickly - so it seems ammonium sulfate may be the best route.
Now I didn't know NaHCO3 could be mixed with ammonium sulfate to produce ammonia + CO2, I didn't think it was strong enough for some reason.
I think I have the equations balanced correctly
2(NaHCO3) + (NH4)2 (SO4) -> 2NH3 + 2CO2 + 2H2O + Na2SO4
Na2CO3 + (NH4)2 (SO4) -> 2NH3 + H2O + Na2SO4
2(NaOH) + (NH4)2 (SO4) -> 2NH3 + 2H2O + Na2SO4
Now my biggest question is how to do this as I suspect mixing these in dry form would produce gas and heat and it should produce enough water to keep
the reaction moving forward. But mixing 200g or reagents might make too much gas too quickly to be useable and adding more slowly can be tedious, so
I'm wondering if doing this in an aqueous solution would be more plausible and if the NH3 would remain in solution, given enough water (cold) to
absorb the NH3. Then the solution could be heated to boil off the NH3 and then capture the gas in an ammonia absorption setup.
Has anyone ever done this in an aqueous solution and then boiled it off? Is there any reason that this shouldn't work?
The other option is to just heat the ammonium sulfate and reduce it to ammonium bisulfate which may be also used with a base to make NH3 but at a
greater cost per mole of NH3 produced.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Won’t you get a lot of ammonium carbonate/bicarbonate?
I’d just use an ammonium salt plus NaOH or KOH personally.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I have never used NaHCO3 for this. NaOH is cheap enough here.
I have found the easiest way to control the reaction is to add the dry reagents to a flask and mix roughly. NH3 is produced straight away but not
quickly at first. Then adding drops of water with an addition funnel lets you control the reaction rate.
Doug's Lab has a nice ammonia video.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I would load up a flask with solid base (NaOH, KOH, CaO) and drop in a saturated solution of ammonium salt. There are some losses due to the product
dissolving in the water. Heating the final solution drives off most ammonia.
In this way I recovered the ammonia from the quite contaminated mother liquor of a recrystalization of several kilograms of ammonium salt
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Garden lime is much cheaper than any previously mentioned base in this thread.
Can anyone see a problem with this.......?
Ca(OH)2 + (NH4)2SO4 --> CaSO4 + 2NH3 + 2H2O
Calcium Hydroxide + Ammonium Sulphate --> Calcium Sulphate + Ammonia + Water
Both ingredients can be bought in 50kg bags at the local farm store
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Corrosive Joeseph  | Garden lime is much cheaper than any previously mentioned base in this thread.
Can anyone see a problem with this.......?
Ca(OH)2 + (NH4)2SO4 --> CaSO4 + 2NH3 + 2H2O
Calcium Hydroxide + Ammonium Sulphate --> Calcium Sulphate + Ammonia + Water
Both ingredients can be bought in 50kg bags at the local farm store
/CJ |
Good point. I didn't think about Ca(OH)2 b/c it doesn't go into solution, but dripping the liquid sulfate in should work I would think.
Edit: I just tested this and it works very well!
My question is again making an aqueous solution. Could I get 27%+ NH3 solution if I made a fairly concentrated ammonium sulfate solution, then added
the calcium hydroxide? The water would absorb the gas, then it could be heated to liberate it? The reason I ask is because not everyone has the
setup to do slow additions AND capture the resulting gas. This is more complex than a simple setup and would require at least a 2 neck flask.
[Edited on 4-14-2018 by RogueRose]
[Edited on 4-14-2018 by RogueRose]
[Edited on 4-14-2018 by RogueRose]
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I just added 1 mole each of calcium hydroxide and ammonium sulfate in 300ml of water (excess to dissolve sulfate and hopefully retain the NH3). I
added the hydroxide to the solution and there was little gas coming out of the solution, about as much as one would expect from a cleaning compound,
so it seems that it does absorb quickly into the water and I feel this is good news as it could be filtered off and a strong solution be made directly
from this reaction.
Can anyone see a reason why this method wouldn't work, maybe add excess hydroxide to ensure complete transofmation of the sulfate and then filter the
liquid getting a relatively strong and clean ammonia solution?
this seems to be an extremely inexpensive way to generate large amounts of ammonia at about $20 total for 50lbs of each.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Calcium sulfate is slightly soluble in water so it may affect some reactions if you just filter the ammonia solution without further purification.
Cody'sLab made a video on making ammonia from calcium hydroxide and ammonium sulfate:
https://www.youtube.com/watch?v=LiJ3Z8a6Ld0&t=0s
[Edited on 14-4-2018 by Plunkett]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
This reaction doesn't work with carbonate or bicarbonate. You'll simply get a lot of ammonium carbonate depositing on every surface the gases pass
through and if you bubble it into water you get the same result only in solution.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Amos  | | This reaction doesn't work with carbonate or bicarbonate. You'll simply get a lot of ammonium carbonate depositing on every surface the gases pass
through and if you bubble it into water you get the same result only in solution. |
Hmmm. IDK where I read that both bicarb and carbonate work for this. I just read it yesterday. I think it was in an ammonia generation thread on
this board. I'll have to give it a test and see what happens. Either way, after trying the Ca(OH)2 it makes little sense to use the others since
this works well. I'm going to try to make a fair amount this way.
IDK if I can dissolve ammonium sulfate in an ammonia solution, so I can get a higher concentration of ammonia. So I'd do a first pass with say 1
mole per 300ml, then filter, then dissolve another mole of ammonium sulfate in the filtrate and then add a mole of hydroxide - hopefully getting 2x
the concentration. Repeat until saturation.
Does anyone know how many moles of NH3 can be dissolved in water? It looks like Sigma sells a 28-30% which is supposed to be 14.8 molar solution but I
always get confused at concentration vs molar amount (is that 14.8 moles in 1L, 1gal, or what..)
https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/...
I've only tried dissolving NH3 into water once and used a 250ml grad cylinder (figured taller was better) and an air stone. I'm thinking of using a
2-4ft PVC pipe and put the stone at the bottom that way the bubbles have longer to travel and be absorbed before reaching the top. IDK if that is
over-kill or useful. I've seen people use plain beakers but don't know how much is lost.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Vacuum filtering ammonia solution - harmful to pump?
Is it a bad idea to vacuum filter a concentrated solution (or even mild to low) of ammonia? I just ran my CaSO4 & ammonia through my filter and I
noticed that there was almost no gasses coming out of the exhaust of the pump. I think this is because the gasses in the flask were ammonia and when
they came into contact with the oil, they were absorbed thus very little was being expelled in the exhaust side of the pump, only the very little air
that came along.
I don't have much experience with vacuum filtration and even less when it is anything other than a non-volitile solution (basically water & salt
solutions).
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RogueRose  |
Does anyone know how many moles of NH3 can be dissolved in water? It looks like Sigma sells a 28-30% which is supposed to be 14.8 molar solution but I
always get confused at concentration vs molar amount (is that 14.8 moles in 1L, 1gal, or what..) |
The maximum concentration you can get depends on the temperature you make/store it at, and ammonia slowly escapes from solution over time so if you
want really concentrated ammonia it is best to make it fresh. Here is a solubility curve for ammonia taken from engineeringtoolbox.com.
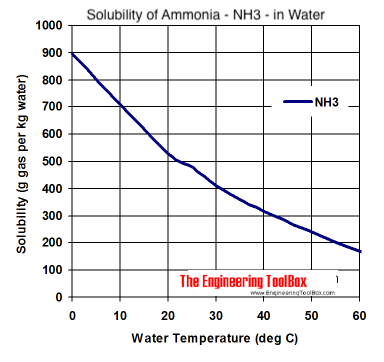
Molarity is moles of solute per liter of solution, e.g. 1 L of a 14.8 M NH3 solution contains 14.8 moles of NH3.
[Edited on 14-4-2018 by Plunkett]
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
So I'm getting what I beleive is an add reaction while trying to boil off the ammonia. I have a flask with a stopper in it and a hose barb coming out
of the stopper. A hose runs from the barb over to the air stone that is in a 250ml graduated cylinder with ice cold water.
My ammonia solution has 11 mole (17g/mole = 186g) of NH3 and 4 5.5 moles of CaSO4 (136g/mole = 748g). The volume of the solution is about 1200ml
including the ammonia and CaSO4.
I boiled in a water bath and I get about 10 minutes of very vigerous bubbles coming out of the stone. Then it dies down a lot (water still a rolling
boil). The solution is a rolling boil around the edges but no gas comes over. I swirl the solution a few times, gas comes over and boiling starts
again for a minute or 2. I did this maybe 10x and then got tired of the water and switched to oil.
Now I got everything heated up and the entire solution (not just edges of flask) are at a rolling boil but nothing is coming over, I see no bubbles.
I'm standing back and waiting for the stopper to blow out thinking there is a clog or something. I notice the grad cylinder is about to overflow and
realize that liquid is coming over. I'm not using a condensor BTW (had one on before but didn' think it was necessary with ammonia) I pull out about
30ml of liquid and smell & taste some wet residue on fingers to see if it has taste yet - very potent. I had tasted some of the water in the grad
cylinder (just a little dampness on finger not even 1/20th of a drop) when switching to oil and it didn't have any bite, so I thought nothing was
being absorbed. Now it is extremely strong and the volume has increased a total of about 70ml by time I pull it off the stove.
My the time I pulled it off the stove, almost the entire flask was filled with bubbles from a very strong boil, so it is clear that there was plenty
of heat to drive off the ammonia, but it seems that it is passing water over at the same time, which seems counter-intuitive based on the temp curve
for holding ammonia. It feels like I'm distilling azeotrope ethanol or something.
So it almost seems like I'm distilling over an azeotrope or something. Yesterday I tried 100g of Urea under heat with the same setup and ran it for
about 10 minutes until it started to pass water over and stopped. The water had a VERY strong ammonia smell, stronger than store bought cleaning
solution (say 3-5%) but I don't think I made a lot during this time. I need to weigh the remaining urea and see how much passed over.
I just checked the solution in the flask after it cooled down and it is still extremely strong, it actually seems stronger than when it started as far
as smell goes, IDK if the act of heating it allowed it to react some of the Ca(OH)2 that wasn't reacted before or what, but the vapors are much
stronger than when I started and the solution from the grad cylinder are very strong as well.
What I don't understand is why the gas stops coming over and the solution boils (212F) in the flask while it still has LOTS of NH3 in it. I'm
wondering if the CaSO4 is holding it in there some how.
I'd like to filter off the CaSO4 but I'm worried about pulling NH3 through the pump and getting NH3 in the oil as when I filtered some before, I
noticed that no air was coming out of the "exhaust" port of the vacuum pump so I think the NH3 was absorbing into the oil possibly. Has anyone vacuum
filtered ammonia solution and know if this can mess up a pump? I have no alternative, no aspirator nor good pressure for it.
This is much more difficult than the threads have made it seem.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
What about using your flask and boiling water as a vacuum source? Boil water in the flask, stopper the flask, connect the hose barb on the stopper to
you vacuum filtration system, and let the water cool. It would be incredibly inconvenient, but if you are concerned about your vacuum pump it might
be a one off alternative.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Plunkett  | | What about using your flask and boiling water as a vacuum source? Boil water in the flask, stopper the flask, connect the hose barb on the stopper to
you vacuum filtration system, and let the water cool. It would be incredibly inconvenient, but if you are concerned about your vacuum pump it might
be a one off alternative. |
I'm using the pump but only cycling it every 10 mins or so and only until I see the filtrate bubble from lack of pressure and the NH3 gassing - then
stop. It works and isn't the best for the pump, but it will work for the small amount I have.
I forgot how hard CaSO4 is to filter. I just got done filtering some MgCO3 and it was about 100x easier and is similar consistency. IDK why there is
such a difference when filtering the two.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I haven't been able to find a solubility chart for ammonia at higher temperatures, but I did find a quote from another sciencemadness thread | Quote: | | Drive out ammonia using heat (not boiling), and redissolve in small amount of cold water. Problem is that at 100C water still holds 7% ammonia, which
is more than household ammonia (but janitorial ammonia is 10%). |
http://www.sciencemadness.org/talk/viewthread.php?tid=23666&...
No source was given for this, but if it's true, it would explain why the solution boiled before all of the ammonia had been driven off. I don't know
if it technically counts as an azeotrope, but it would definitely have a similar effect on distillation/reabsorption of ammonia. Other than filtering
the calcium sulfate out, I suppose you could just distill the whole reaction mixture until most of the water (and ammonia too) has came over. Of
course, that would take a lot of time and energy. Another thing to do would be to use less water. It doesn't have to be enough to dissolve all the
ammonium sulfate at once, I wouldn't think. If even a bit of it dissolves, it will react, creating (mostly) insoluble calcium sulfate, ammonia, and a
bit more water. Of course, now you have to figure out a way to capture all the ammonia quickly, since it will now outgas as the reaction precedes.
Maybe you could put a beaker full of water upside down in a bigger container of ice water, then put the bubbling tube underneath it to trap the
ammonia until it can dissolve. I suppose there are ways to capture NH3 without water, but the solutions I thought of (gas bag, compressed cylinder,
condensing using low temperature or very high pressure) would be hard to do safely in a home lab.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Vomaturge  | I haven't been able to find a solubility chart for ammonia at higher temperatures, but I did find a quote from another sciencemadness thread | Quote: | | Drive out ammonia using heat (not boiling), and redissolve in small amount of cold water. Problem is that at 100C water still holds 7% ammonia, which
is more than household ammonia (but janitorial ammonia is 10%). |
http://www.sciencemadness.org/talk/viewthread.php?tid=23666&...
No source was given for this, but if it's true, it would explain why the solution boiled before all of the ammonia had been driven off. I don't know
if it technically counts as an azeotrope, but it would definitely have a similar effect on distillation/reabsorption of ammonia. Other than filtering
the calcium sulfate out, I suppose you could just distill the whole reaction mixture until most of the water (and ammonia too) has came over. Of
course, that would take a lot of time and energy. Another thing to do would be to use less water. It doesn't have to be enough to dissolve all the
ammonium sulfate at once, I wouldn't think. If even a bit of it dissolves, it will react, creating (mostly) insoluble calcium sulfate, ammonia, and a
bit more water. Of course, now you have to figure out a way to capture all the ammonia quickly, since it will now outgas as the reaction precedes.
Maybe you could put a beaker full of water upside down in a bigger container of ice water, then put the bubbling tube underneath it to trap the
ammonia until it can dissolve. I suppose there are ways to capture NH3 without water, but the solutions I thought of (gas bag, compressed cylinder,
condensing using low temperature or very high pressure) would be hard to do safely in a home lab. |
Thanks for the quote from the other thread! I knew there was something that didn't seem right with the ammonia boiling over. The thing is, I have
read MANY times that to make concentrated ammonia all you have to do is heat household ammonia and run the gas into water or another solution of
ammonia. Well, I guess that is another "common" knowledge idea that isn't quite correct.
I thought about making the solution more concentrated by using less water but ran into some road blocks, similar to what you wrote.
I am going to try ammonium sulfate and NaOH and try doing it dry but I'm not sure how to capture the gas fast enough.
I'm wondering if the 7% can be lowered by using vacuum but then you have a gas coming over and also pulling vacuum on a solution that off-gasses
easily, so I don't think that will work.
Baring all this, I am going to try just heating the sulfate to decomp point as that will give pure gas without the problems of addition of more
reagent. I'd really like to do this with urea but IDK at what point the reaction stops. I see the liquid boiling and gasses coming over, then a lot
of water vapor & milky white isocyanic acid(or biruet) starts clogging the tubing.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I just remembered another option, from when I had a few ammonia questions of my own. I basically wanted to know if all the byproducts from pyrolysis
of urea were toxic (as in, more so than ammonia itself.) Anyhow, a bunch of members suggested ways to make ammonia, and this was one of the methods:
| Quote: | | Thermal hydrolysis of urea, that is heat with water, is a better option. It's slow but steady. Depends on how much you need at a time. Pyrolysis is
a mess chemically and practically. Urea decomposes with co evolution of CO2, so that needs to be taken in to account. The sulphate method is a good
lab scale preparation though. |
By Chemetix
http://www.sciencemadness.org/talk/viewthread.php?tid=80659&...
I don't know how well that will work in this case (sounds like you might still create deposits of ammonium carbonate in your apparatus) but it might
be worth a shot.
Also this: | Quote: | | Rather than pyrolyzing your urea and making a huge mess, you can simply add it to a solution of NaOH to generate ammonia quickly and cleanly (not as
fast as an ammonium salt, but still a good steady rate), with sodium carbonate as your only byproduct. |
By
Texium.
http://www.sciencemadness.org/talk/viewthread.php?tid=80659&...
I don't know if that would produce as controlled of a gas release as a boiling solution, but it is just another of the ideas I was given. I didn't
share them earlier, because I thought they seemed very similar to the things that had already been discussed. But they might have some key differences
that make them easier to manage.
Edit: I thought you could distill weak ammonia too, until I found that other quote. I suppose you could distill it to get rid of non-volatile
impurities like detergents or scents (or calcium sulphate) but it seems like you can't boost the concentration.
It's ironic that ammonium sulphate, and urea are made from ammonia, and yet ammonia solution costs more. I suppose it's because few people use
concentrated solutions in bulk. The farmers who use ammonia by itself use the "hard to do safely in a home lab"(And also regulated as a drug precursor
in some places) anhydrous gas, and they probably get it pretty cheap.
[Edited on 15-4-2018 by Vomaturge]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Depending on what one is going to attempt with the ammonia water, perhaps consider living with the ammonia/CaSO4 aqueous suspension.
For example, applying UV light to say CH3OH/H2O/NH3/CaSO4 with possible reactions:
CH3OH + UV + M --> .CH3 + .OH + M
NH3 + .OH --> .NH2 + H2O
.CH3 + .NH2 --> CH3NH2
.NH2 + .NH2 --> N2H4
........
would probably produce higher product yields with the suspended particle matter M (CaSO4) to reflect and diffuse the UV light! A quick search suggests
this also, see for example "Effects of suspended sediments on photolysis rates of dissolved pollutants", at https://www.sciencedirect.com/science/article/pii/0043135479... . If lower yields are observed, this may be due to specific shielding effects (see
https://books.google.com/books?id=8R_F5MC0Pa4C&pg=PA151&... ).
The reputed benefits of diffused light (which I noted in a recent thread comment, see http://www.sciencemadness.org/talk/viewthread.php?tid=81813&... ) as a source of short lived radical reagents may be sort of analogous, in my
opinion, to the benefits ascribed to increased surface area contact and kinetics, like from stirring.
[Edited on 15-4-2018 by AJKOER]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by RogueRose  | | Now my biggest question is how to do this as I suspect mixing these in dry form would produce gas and heat and it should produce enough water to keep
the reaction moving forward. But mixing 200g or reagents might make too much gas too quickly to be useable and adding more slowly can be tedious, so
|
...BTW anyone know what happens if most concentrated alkali hydroxide is added dropwise through an addition funnel into a heated flask of the dry
sulfate?
Or when most hot concentrated sulfate is added to dry alkali?
[Edited on 15-4-2018 by S.C. Wack]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The OP is clearly well acquainted with this, so can give a first-hand and recent account of how this proceeds.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
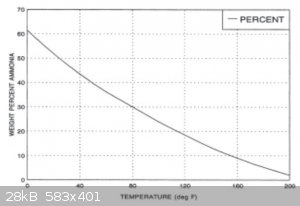
Source: http://web.iiar.org/membersonly/PDF/CO/databook_ch2.pdf p.27
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Well this experiment has been well worth the time and frustration even if I only learned that water will retain 7% ammonia even at boiling! This
seems to be in direct contradiction of the MANY claims that a way to get more concentrated ammonia is to buy retail ammonia solution and heat it to
drive off the ammonia but since the average composition is 3-5% (a few professional grades are 10% but it is about 3x more expensive) this isn't
possible. I wonder how many people have posted this method and never tried it. I've seen this as the primary method for "ammonia generators" but that
all seems to be BS now (at least the generation part).
As for the question about what I intended to do with the ammonia, I was planning on playing around with some copper and nickel complexes so it seems
that pure ammonia is ideal in that case. I also wanted some to have on hand that I know doesn't have soap or detergents in it.
I did 3 more tests in test tubes. One was adding NaOH to ammonium sulfate. The problem is that it produces gas for a short period, along with some
heat, then stops. It needs agitation to continue and it seems the water produced by the reaction falls a little short to keep the reaction moving
forward. IDK if heating the mix would help? I can see that this method is also far from ideal and there will be a good bit unreacted in any setup.
I also added some drops of water, though I wanted to avoid this as we have seen it so readily absorbs ammonia. Upon adding a few drops of water, the
gas stopped evolving from the mix, which I assume means it was being absorbed.
I also looked at urea again and now I've looked into this more it seems more plausible. When heated 3 urea molecules combine to produce isocyanic
acid at ~129g/mole and 3 molecules of NH3 at 17g/mole. Urea being 60g/mole that is about a 28% yield of NH3 from urea. In this reaction there are no
other gasses produced so that is good and the reaction takes place at a reasonable temp of between 212 and 400F. When the urea is heated (flame has
been best method for this I've found though others would work if adequate BTU is used) it will turn clear and produce NH3. Once it melts and turns
clear, you need to keep heating at a temp that will continue to decomp the urea or else you get side products that really mess things up it seems. If
heating strongly then the reaction is straight forward, simple and complete. When the melted boiling solution turns white, that means all the urea
has reacted, all the NH3 is gone and the isocyanic acid has formed and the reaction will stop. Remove from heat and try to remove the white substance
ASAP as it is very difficult to remove later and it doens't dissolve very well even in boiling water.
So now I need to figure out what to do with the isocyanic acid....
I tried heating ammonium sulfate to decomp in test tube over propane torch. It is a VERY messy and troublesome process as the compound doesn't heat
uniformly, doesn't really melt at the temp and tends to pop and spit hot salt out of the test tube. It also produced some gas other than NH3 and was
white-ish. I suspected it was CO2 of H2O vapor but I was working with a dry salt and decomp shouldn't have produced any CO2 and only produces H2O at
high temps after the bisulfate has been formed and when it decomposes at which point SO3 also forms, so this is not an ideal method of production if
the process can't be limited to the first step of decomposition to the bisulfate (between 212F and 400F as well).
Here is an interesting article on ammonium sulfate decomposition.
http://celbar.com/wp-content/themes/celbar/assets/pdf/Ammoni...
Attachment: Ammonium Sulfate.pdf (24kB)
This file has been downloaded 388 times
Now as far as adding NaOH to urea, I haven't tried that but I suspect it would react similarly as adding hydroxide to ammonium sulfate and not sure
what it would produce but I suspect it would make sodium carbonate while evolving NH3 & CO2.
I never expected that it would be so difficult to produce pure NH3 at a constant rate without having all the other complications and co-products.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 285
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
Just two more thoughts on this subject.
First of all, I never found detailed information on isocyanic acid, but one urea MSDS noted it as a decomposition product with a 10 minute permissible
exposure of .07 milligrams per cubic meter. Hopefully that's a misprint, but it might be a reason to be careful when heating urea.
Secondly, I am not completely sure whether water retains 7% ammonia at the boiling point. That goes against what is said in the pdf Plunkett cited.
Also, the guy who claimed that, Gammafunction, didn't give any citation. Still, if it were true, it would explain why your ammonia solution still
retained ammonia after vigorous boiling.
Just take everything I say here with a grain of salt. It's mostly based on things I have read from not-so-authoritative sources I do have some weak cleaning ammonia. If I get a chance to try boiling gas off of it,
I'll post the results. Worst that could happen (barring some accident), I would have some non-soapy ammonia solution. I do have some weak cleaning ammonia. If I get a chance to try boiling gas off of it,
I'll post the results. Worst that could happen (barring some accident), I would have some non-soapy ammonia solution.
Anyhow, good luck getting some pure ammonia, and experimenting with complexes!
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I'd like to know how fast ammonia gas is absorbed into water. When I was heating my ammonia/CaSO4 solution I used an aquarium air stone and the
bubbles were pretty small. They flowed about 10" or so up the graduated cylinder in water that was 40-70F. I noticed a fair amount of bubbled
reaching the surface but when I put my nose to the top I smelled very little ammonia, probably just what was in solution, so I'm wondering what these
bubbles that reached the surface were.
I had mixed ammonium sulfate and Ca(OH)2 in water, then heated in a 2L flask and was getting these bubbles 30-40 minutes into the reaction, which I
expected as NH3 bubbles, but no other gasses. I guess they could be air that is in the flask (I started with ~1400ml of solution) but after that
amount of time I would have expected that all the air would have passed over by that point and when I opened the flask it was STRONG ammonia (I would
say saturation or near 95%). Considering that the levels of detectability (odor threshold) are 5-50 ppm then these bubbles must have very little
ammonia in them for the smell to be so faint.
I'm going to be passing gas through a solution again and I want to make the most of the gas produced, so I need to figure out how long of a cyinder I
need for the NH3 to be absorbed. I'm planning on using either PVC pipe filled with ammonia solution or possibly an old flourescent light tube (the
4ft long & 1 - 1.5" diameter) surrounded with ice water to keep it as cool as possible. If anyone has any other ideas on a good method for this,
I'd appreciate hearing it. I just need to know how "deep" the absorbing solution/water should be.
I've attached a document that has a lot of statistics about ammonia, it is very extensive at 32 pages and I hhaven't had a chance to look through all
of it, but others might find some good information in it.
Attachment: Ammonia.pdf (1.3MB)
This file has been downloaded 958 times
[Edited on 4-16-2018 by RogueRose]
|
|
|
| Pages:
1
2 |