JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Modified Wolff-Kishner
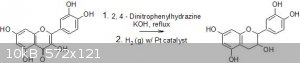
TL;DR: Will the first transformation in the attached picture work to remove the C=O?
Last semester I did a project analyzing red wines for levels of resveratrol, quercetin, and polydatin via HPLC and we found a spike that we suspected
was a form of catechin. Due to time restraints and deadlines we were unable to go back perform further tests to confirm our suspicions but I was
allowed free reign with the leftover reagents and I'll have access to the university lab over the summer before I leave for MN. With that backstory
out of the way here's my question:
Is it possible to perform the Wolff-Kishner reduction with a hydrazine derivative rather than just plain old hydrazine?
Another group had left over 2,4-Dinitrophenyldrazine that I wanted to try and use to reduce our excess quercetin's C=O in a transformation from
quercetin to (+/-)-catechin. (I'm planning on reducing quercetin's C-C double bond with H2 and a Pt catalyst) I wasn't sure if this was possible since
generally you're taught that the driving force behind the W-K is the removal of N2 gas. Based on that, and the mechanism, even if I got rid of the
nitro groups to just phenylhydrazine I don't know if it might work.
I started going through the ACS's articles and an old 1949 paper [1] suggested that substituted hydrazines would actually form new C-C bonds upon W-K
conditions, but a newer undergraduate lab to teach the W-K [2] uses a hydrazine derivative without fear of such side reactions.
Has anyone tried similar reactions before with any success or know more about the theory to give some suggestions? Thanks in advance!
It's been a while since I've been on SM, all the missed content will make for great reading on my flight 
References:
1) J. Am. Chem. Soc., 1949, 71 (4), pp 1356–1358
DOI: 10.1021/ja01172a062
Publication Date: April 1949
2) J. Chem. Educ., 2016, 93 (5), pp 949–952
DOI: 10.1021/acs.jchemed.5b00954
Publication Date (Web): February 3, 2016
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
See this mechanism: https://www.organic-chemistry.org/namedreactions/wolff-kishn...
As long as there's some water or other proton source around, I expect that the reduction will proceed normally.
[Edited on 5-18-2018 by Metacelsus]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Why not make some hydrazine hydrate. I show how in another thread. FYI, here's my experience with the Wolff-Kishner, Huang-Minlon version: https://www.sciencemadness.org/whisper/viewthread.php?tid=68...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
tl;dr: no, but you're not screwed.
Step 2 in your attached picture says "H2/Pt" after using dinitrophenylhydrazine. I assume this is an important part of the reduction procedure. In
particular, note that benzylic C-N and C-O bonds can usually be cleaved by hydrogenation whereas aliphatic ones are not. I think they just pop off the
hydrazone with platinum. Note also the double bond is reduced.
In the Journal of Chemical Education paper, a hydrazinecarboxylate ester is used as a hydrazine substitute. Uniquely, this hydrazine
derivative will decarboxylate to release free hydrazine under Wolff-Kishner reduction conditions. This is why this particular modified hydrazine is
suitable for ketone reduction.
One interesting possibility might be to make the hydrazinecarboxylate ester yourself, which I suspect might be possible by performing a Hofmann
degradation of urea in tert-butanol or another alcohol. This should generate intermediate isocyanatoamine H2NNCO which reacts with the alcohol to give
the hydrazinecarboxylate ester.
[Edited on 18-5-2018 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
DNPH and it's aldehyde/ ketone derivatives are unstable in alkaline environments.
Heaven knows what you would get from the proposed reaction.
|
|
|
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
Thanks for the replies!
@Metacelsus - The third to last step in that mechanism though, where the di-imine (I think thats the nomenclature?) has a proton pulled off by
hydroxide and the electrons fall back on the nitrogen which later kick out N2 from the carbon is the issue. In trying to use a hydrazine derivative I
would have a phenyl ring there not a proton. I didn't think the base could just pull that off?
@Magpie - I was hoping I could limit the project to only using leftover reagents from my and the other groups over the semester, I'll check with the
lab tech to see if they have any hydrazine hydrate or sulfate (from which I'll follow your prep to the hydrate) on hand.
@clearly_not_atara - Ah, so that specific hydrazine derivative is required, i.e. the carboxylate ester. I didn't even think of that decarboxylating,
thanks for clearing that up. The hydrogen gas and platinum in the second step is solely to reduce the C-C double bond. The first step is what I'm
trying to work out to remove the carbonyl on my way to catechin. I'll look into that Hofmann degradation route to the carboxylate ester.
@unionised - Damn, well it looks like I'll try to see what else they have on hand to reduce the carbonyl. Maybe a clemmensen reduction? I think they
have some mercury metal in the storeroom. Or the hydrazine carboxylate ester route atara helped me understand.
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
DNPH fragment contains 2 nitro-groups which will be reduced at first by H2/Pt. Then C=N and N-N bonds (of the C=N-N fragment) can be reduced to obtain
the corresponding amino-derivative (CH-NH2). And only at the last step it can be reduced to CH2.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by JnPS  | @unionised - Damn, well it looks like I'll try to see what else they have on hand to reduce the carbonyl. Maybe a clemmensen reduction? I think they
have some mercury metal in the storeroom. Or the hydrazine carboxylate ester route atara helped me understand.
|
I'm not sure if you'd have problems with that ether getting cleaved or not under clemmensen reduction conditions. However, if you're dead set on
getting rid of that carbonyl oxygen, how about Monzingo reduction? As a bonus, the Raney nickel would (probably) hydrogenate the double bond.
[Edited on 5/21/18 by Melgar]
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
You can try reduction via tosylhydrazone formation and its NaBH4 reduction, see the next link:
http://orgsyn.org/demo.aspx?prep=CV6P0062
|
|
|