Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
von Braun Cyanogen Bromide Reaction alternative
I'm looking for an alternative to von Braun Cyanogen Bromide reaction , that uses less/non toxic reagents.

I've found an alternative option (Comprehensive Organic Functional Group Transformations, Volume 6, p444) but it utilizes phosgene what is even more
toxic.

|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
The only know (by me) alternative to do this kind of N-dealkylation is using alkyl or aryl chloroformates, which are all as toxic as BrCN, followed by
heating with methyl alcohol at reflux.
[Edited on 28-5-2018 by Chemi Pharma]
|
|
|
Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemi Pharma  | The only know (by me) alternative to do this kind of N-dealkylation is using alkyl or aryl chloroformates, which are all as toxic as BrCN, followed by
heating with methyl alcohol at reflux.
[Edited on 28-5-2018 by Chemi Pharma] |
Thanks! I've just found this article based on your advice.
New, Useful Reactions of Novel Haloformates and Related Reagents
Article in Pure and Applied Chemistry 60(11):1715-1724 · January 1988
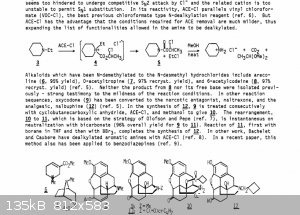
Attachment: 10.1.1.567.5857_New, useful reactions of novel haloformates.pdf (792kB)
This file has been downloaded 438 times
See also :
A new reagent for the selective, high-yield N-dealkylation of tertiary amines: improved syntheses of naltrexone and nalbuphine
R. A. Olofson, Jonathan T. Martz, Jean Pierre Senet, Marc Piteau, and Thierry Malfroot
J. Org. Chem., 1984, 49 (11), pp 2081–2082
DOI: 10.1021/jo00185a072
Publication Date: June 1984
[Edited on 28-5-2018 by Mush]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Thank you either @Mush for the paper.
I didn't know acetaldehyde, phosgene and catalytic PTC with chloride ion afford alfa chloroethyl chloroformate, which is really an useful reagent.
Do you think the easier to achieve tert butil ammonium chloride (TBAC) could be used instead PhCH2N(nBu)3Cl (BTBAC), in this synthesis ?
Edited: TBAC - tetra butyl ammonium chloride, no tert butil. I apologize. Thanks @Outer for the correction. Tert butyl alcohol were in my head.
[Edited on 28-5-2018 by Chemi Pharma]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
TBAC is tetra butyl ammonium chloride, and not tert-butyl ammonium chloride. Yes, it can be tried as well.
|
|
|
Mush
National Hazard
   
Posts: 632
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
New Methodology for the N-Demethylation of Opiate Alkaloids
Zemin Dong, and Peter J. Scammells*
Department of Medicinal Chemistry, Victorian College of Pharmacy, Monash University, 381 Royal Parade, Parkville, Victoria 3052, Australia
J. Org. Chem., 2007, 72 (26), pp 9881–9885
DOI: 10.1021/jo071171q
Tetrasodium 5,10,15,20-tetra(4-sulfophenyl)-porphyrinatoiron(II)
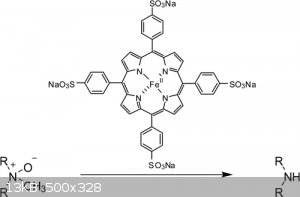
Attachment: New Methodology for the N-Demethylation of Opiate Alkaloids.pdf (87kB)
This file has been downloaded 300 times
|
|
|