DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
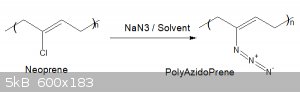
Poly-Azido-Prene from Neoprene
So similar to the synthesis of:
Glycidyl chloride polymer > GAP
and PVC to PVazide.
What about Polychloroprene to polyazidoprene?
Here is a possible route? any thoughts?
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
It would make sense seeing the chlorine as a leaving group. But does neoprene typically exhibit a reactivity to alkali salts? Does that chlorine
remain during crosslinking? I tried to briefly answer these myself, but I couldn't find anything obvious.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by roXefeller  | | It would make sense seeing the chlorine as a leaving group. But does neoprene typically exhibit a reactivity to alkali salts? Does that chlorine
remain during crosslinking? I tried to briefly answer these myself, but I couldn't find anything obvious. |
Thank you for your input. Hmmm, what do you mean by crosslinking? like after curing? Why would the chlorine not remain? thank you !
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Crosslinking = vulcanization, adding other monomers is copolymerization.
Sulfur and heat is the classic crosslinking technology, developed by Goodyear and others back in the 1800s.
Crosslinking with thiocarbamide is a bit more modern, peroxides may be used as well. Ethylene thioureas are effective for crosslinking chloroprene
rubber.
You are interested in this for a composite rocket binder/fuel? With lighter elements than Sulfur for crosslinking, perhaps increasing isp? There is
also UV radiation based crosslinking, tough to do if you've doped the fuel grain with Carbon black to prevent IR penetrating the fuel grain and
accelerating the burn...

[Edited on 5-30-2018 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
I'm not able to get smart about the specifics of neoprene at the moment, but my question was this: what is the process used to vulcanize or crosslink
the base polymer (not the monomer). Other polymer systems use a reactive element of the base polymer to initiate the crosslinking, such as double
bonds. After that is done the base polymer is less reactive and effectively a massive molecule(s). I suppose the author may be considering sourcing
the uncured polymer but will curing still require the chlorine or does it use the double bond?
[Edited on 30-5-2018 by roXefeller]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Bert  |
Crosslinking = vulcanization, adding other monomers is copolymerization.
Sulfur and heat is the classic crosslinking technology, developed by Goodyear and others back in the 1800s.
Crosslinking with thiocarbamide is a bit more modern, peroxides may be used as well. Ethylene thioureas are effective for crosslinking chloroprene
rubber.
You are interested in this for a composite rocket binder/fuel? With lighter elements than Sulfur for crosslinking, perhaps increasing isp? There is
also UV radiation based crosslinking, tough to do if you've doped the fuel grain with Carbon black to prevent IR penetrating the fuel grain and
accelerating the burn...
[Edited on 5-30-2018 by Bert] |
we can hydroxylterminate the polychloroprene to form HTPCP, now if we add azide groups, it'd be HTPAP. Then it could be cured with isocyanates.
However, straight up polyazidoprene does not need crosslink to cure. cyanide groups can cure it, for example a small amount of polynitrile will cure
with the azide groups. the CN N3 forms a tetrazole ring.
[Edited on 30-5-2018 by DubaiAmateurRocketry]
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
so, any other input if this reaction might work ?
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
It will not work. Vinylic halides are unreactive in SN1/SN2 type reactions which is what is being proposed here.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by UC235  | | It will not work. Vinylic halides are unreactive in SN1/SN2 type reactions which is what is being proposed here. |
How come PVC works? Thanks!
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Despite the name, the chlorine atoms on PVC are not vinylic halides, but alkyl halides, which are quite reactive.
|
|
|