aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Vicine
For over a year i've been trying to extract vicine from fava beans.
Today i picked up the latest tiny plastic pot of brown goo with the intention of throwing it out as yet another total failure.
When the pot was squeezed, it was crunchy.
Pouring off the nearly black supernatant (which had a crust on top) revealed crystals !
 

There isn't much, but at least there is something - finally !
Now for the scary part - recrystallising this micro-gramme amount, separating the convicine then recrystallising it again.
Happy days !
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Vicine? You got a grudge against somebody with a glucose-6-phosphatase deficiency?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quite the opposite.
Research is somewhat hampered by the $35,000 per-gramme cost of the stuff at analytical purity.
Marquardt et al's process is a bit beyond an amateur in the form it states.
Great googling btw.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Do you have any Craig tubes?
They're just great for re-crystalizing small quantities with minimal loss. (with a centrifuge, but you've got one of those.)
They use them in microscale kits (that 14/10 stuff), but they must be available alone.
Did you find an MP for that stuff?
How are you planning to characterize it?
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Have you taken a look at DOI: 10.1002/jsfa.2740361210 ? The extraction looks fairly straightforward.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Did you mean to say: "Great Googly Moogly"?
Stop being rude to people.
Folks from where I went to school, know what Favism is.
$30,000 a gram?
That might be worth the trouble.
https://www.sigmaaldrich.com/catalog/product/sial/78260?lang...
Yup!
[Edited on 6-6-2018 by zed]
[Edited on 6-6-2018 by zed]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
No, i do not - did not even know they existed - thanks. Really good info.
I now suspect that Craig tubes are what Marquardt et al used when they said 'crystals recovered by centrifugation'.
Quote: Originally posted by UC235  | | Have you taken a look at DOI: 10.1002/jsfa.2740361210 ? The extraction looks fairly straightforward. |
Thanks for the reference. Yes, i've read that also.
Everything looks easy until you try and do it.
Seems to get harder at that point, especially when there is no 'No. 245 Carter Seed Cleaner' nor a 'Type 132 MP' on hand.
I was congratulating you on your search skills, not trying to be rude.
The probability of addressing anyone with prior knowledge of vicine is vanishingly small, which i offer as an excuse for assuming it was googled.
Closer to $40,000. Around 35,000 euros a gramme.
... not that the production of vicine could ever make anyone anyone some actual money.
It can only really be used in research, where milligrammes are more than sufficient.
It could find applications - i only found it by accident when NurdRage was making Pyrimethamine back at the NaCN stage.
Seemed to me that vicine was already mostly what he wanted to make.
If you tried to make and store a whole gramme, chances are that it would have badly degraded before a buyer was found.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Can you state what you did? Very nice that you actually did some chemistry, but for all I know those are just funny looking NaCl crystals.
It would be preferable you explain your experiments in such detail that we could exclude other compounds forming these nice crystals.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Perhaps it is easier to extract Vicine from Bitter Melons? If indeed, it is actually present there.
Now, I love me my Fava Beans, fer eatin', but they are just chocked full of fibrous materials and highly pigmented, seemingly oxidizable other stuff.
Never tasted them until I was 40ish, and I grew my own. Delicious! Sometimes called Windsor Beans, Broad Beans, or English Broad Beans. They were
not a common food on the West Coast of the U.S.A., at least when I was a boy. In California, the Pinto, the Pinquito, and other New World Beans,
reigned supreme.
Is it as it appears? That the toxic part of Vicine is not the nitrogenous ring, but rather the form of glucose it delivers as it is metabolized?
Reminds me of Solasodine (aka Curaderm), of which it is reputed, that it delivers to cancer cells, a type of sugar, that because of the cell's
abnormal metabolism, kills them.
https://www.ncbi.nlm.nih.gov/pubmed/2257540
About Favas in the U.S..... Circa 1984, most people had never heard of them.
https://www.nytimes.com/1984/04/11/garden/a-bean-called-fava...
[Edited on 10-6-2018 by zed]
[Edited on 10-6-2018 by zed]
[Edited on 10-6-2018 by zed]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
The beans and the deficiency that results in favism are discussed in the popular AP Biology suggested class-read book 'Survival of the Sickest', and I
suppose that many have heard of the bean and the disease from this book. This is certainly an interesting extraction, and I would love to hear how you
did it Aga.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | Can you state what you did? Very nice that you actually did some chemistry, but for all I know those are just funny looking NaCl crystals.
|
Very Good point.
I was just getting used to the idea that nobody cared how anything is actually done, with just random words required instead of actual work, or
details.
Over about 20 experiments, the process has been pretty much the same, using Marquardt et al's 1983 paper, but with amateur adjustments/bodges.
First the frozen beans were thawed in water, then separated from their 'testa' by hand.
The separated cotelydons were liquidised in a kitchen food blender.
(several other things were tried, the following was the last attempt)
An NaOH solution was prepared, mixed with acetone and the whizzed-up bean cotelydon mush was stirred by hand with the NaOH solution + acetone for
around 15 minutes.
1 metre of 40mm diameter water pipe was used, with 4 holes drilled at one end to accept two wooden skewers to make a cross, also two holes at the
opposite end to accept supporting string.
A small wad of cotton was blown from the other end to form a plug.
A small quantity (about 100g) of fine sand was then poured into the top of the pipe, with water passed through to ensure that the filtering assembly
was moist.
The filter/pipe was then hung by string from a support.
The bean mush was placed in a towel and hung up so the dissolved material could drip out. This took about 2 days each time.
The pre-filtered green stuff was then put into the pipe-filter and left to filter for about 4 days.
The vast quantity of other stuff in plants makes it a slow process.
Eventually there is a lot of brown liquid in a pot.
Lacking a cyclone evaporator, it just got Boiled down, then ignored for about a month.
The Second time i did all this there were crystals - since then there were none.
That little pot with possibly-table-salt with brown goo in the photo represents a lot as far as i am concerned.
It's some crystals of something after a great many 'no-crystal' experiments.
The separation/recrystallisation isn't going well. It's a very small amount.
That part is also non-trivial it seems.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I know a few people from SM that might offer you NMR analysis of your product, if this is indeed vicine that is quite the success. I will have to look
up that paper myself. I wonder if there's info on the crystallography of vicine, it looks like you've got a pretty consistent and identifiable shape
in those crystals. I'm always interested in these biological extractions.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
What are the masses of the beans and crude crystals?
What solvent u used?
[Edited on 11/06/18 by fusso]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by SWIM  | Do you have any Craig tubes?
They're just great for re-crystalizing small quantities with minimal loss. (with a centrifuge, but you've got one of those.) |
Nope. I had nothing like a Craig tube .... until now. Thanks for mentioning them !
A bit of FreeCAD and ... Tadaaaa !
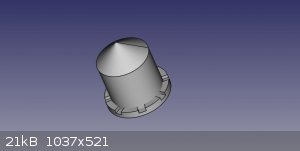
Takes about 15mins to 3D print. Took a while to scale it exactly right in the slicer (Cura) for it to 'just' fit my tubes.
It should be tight enough so that it doesn't leak until it is centrifuged, but loose enough so that liquid leaks out when you spin it up.
Simple test is to fill it with water, fit the cap, then swing it down hard in your hand.
Some drops should leak out.
There's a hole in the bottom to shove a cocktail stick in.
Turned out to be important that the bottom of the tube does not sit in liquid.

STL file to print your own:-
Attachment: craig10.6.stl (54kB)
This file has been downloaded 584 times
[Edited on 23-6-2018 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Awesome ! It Works !
Crystals in brown goo

Transfer to plastic test tube (my glass ones do not have very straight sides - there's a lip)

3D printed in PLA 'Craig' adapter fitted plus cocktail stick

Assembly after about 3 minutes of cranking the handle on my Indian centrifuge

Remarkably the crystals were free in the tube, as if dried overnight.
I just flicked the tube once and they all fell out onto a watch glass.

They still look like brown sugar, and are likely highly contaminated with NaCl.
Maybe that's what they actually are, seeing as the pH was adjusted with NaOH + HCl.
Either way, i'm really impressed with this Craig Tube thing for separating crystals from the mother liquor.
It's just so fast !
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Forgot to mention that it was the Small pot that got whizzed.
Big pot tomorrow, probably 5 batches in all.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
After a lot of cranking the handle on the centrifuge today, all the crystals were separated from the thick brown mother liquor.
Vicine is highly soluble at a pH of 1 wheras convicine is not, so i decided to add 20% HCl to the crystals to separate the two substances at this
point
A tiny portion refused to dissolve, so they should be mostly convicine, certainly not NaCl.
These were separated by decantation and placed in an eppendorf tube, a craig adapter fitted, then centrifuged.

These crystals were washed twice with HCl and recentrifuged, yielding ~100mg of brown crystals.

To the naked eye they appear square-ish.
Under a microscope they have an odd rounded, almost 'organic' look

The HCl portion separated earlier should contain the vicine, so it was filtered, pH adjusted to ~7 with NaOH, then split between 9 plastic test tubes
with caps, which were all placed in a cool box with plenty of ice.
Hopefully crystals will appear, which can then be directly centrifuged out with no need for another transfer.
[Edited on 24-6-2018 by aga]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
How many beans make five?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
About 3kg of frozen beans in this latest batch.
TBH i can't exactly remember.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Congratulations Aga, that's some great work! Do you have anything you plan on doing with them? Perhaps a metal-complex?
|
|
|