Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
2,3-dichloropropene synthesis?
Ok so my target molecule is 2,3-dicholoropropene and i have slightly convoluted way to get there.
Im kinda looking for not only a route to my target that does't require 1,2,3 trichloropropane but also id like to figure out if my reaction could work
in the manner i propose.
My route involves reacting hydroxy acetone with 2 equivalents of thionyl chloride, the first mole would react with the alpha hydroxy group forming the
nasty chloroacetone in situ and a mole of SO2 and HCl, the HCl im hoping could protonate the ketone forming an enol, specifically
1-chloro-2-hydroxypropene.
This i was hoping would then go on to react with another mole of thionyl chloride to prepare 1,2-dichloropropene, which could then be cracked
(isomerised) to form the desirable 2,3-dichloropropene.
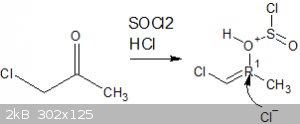
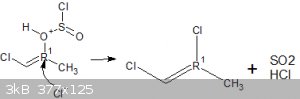
That is what i want to happen however im unsure as to whether it would work as i dont know if that Cl- anion must attack the carbon first followed by
elimination of the leaving group in an intermediate step, or if the leaving group would leave first allowing the Cl- anion to pick up that electron
thats left behind.
From there (assuming it worked) i would like to isomerise that double bond to the more saturated carbon like so.
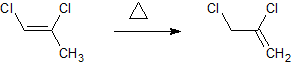
Unfortunately ive no idea how to calculate the enthalpy for such a reaction.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I don't think your planned isomerization is viable. Since you're planning to use thionyl chloride anyway, why not react it with glycerol to form
1,2,3-trichloropropane, and then do an elimination?
From wikipedia:
| Quote: | | 1,2,3-Trichloropropane can be produced via the chlorination of propylene. Other reported methods for producing 1,2,3-trichloropropane include the
addition of chlorine to allyl chloride, reaction of thionyl chloride with glycerol, and the reaction of phosphorus pentachloride with either 1,3- or
2,3-dichloropropanol. TCP also may be produced as a byproduct of processes primarily used to produce chemicals such as dichloropropene (a soil
fumigant), propylene chlorohydrin, propylene oxide, dichlorohydrin, and glycerol.[3] |
[Edited on 6-17-2018 by Metacelsus]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
That isomerization breaks Zaitsev's rule I think. The fact that you want the anti-Zaitsev compound makes this one tricky.
One way to get around that is to start with 3-chloropropyne and add HCl. It might be possible to make this from propargyl alcohol which IIRC can be
made from acetylene + formaldehyde, although you need a different chlorinating agent than SOCl2 I'm pretty sure.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
http://www.orgsyn.org/demo.aspx?prep=CV1P0209
If the bromide is not suitable, then I suspect the chloride would behave similarly.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Yea it seems I applied zaitsevs rule to the enolation without thinking to apply it to the isomerization.
I was hoping to avoid 1,2,3 trihalopropane due to its carcinogenicity, however i suppose a fume hood and respirator would easily mitigate any dangers,
perhaps my fear is unnecessary.
Anyway i shouldn't complain given that the elimination route is significantly more OTC that my method, allyl bromide could be halogenated with bromine
to form the desirable 1,2,3-tribromopropane.
Thanks for the replies guys.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
I'd be shocked if the target product is somehow not carcinogenic as well. Probably quite the lachrymator too. A fume hood is in order.
|
|
|