| Pages:
1
2 |
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
Fumes from CuCl2 + Al Reaction
I recently preformed an experiment which involved reacting anhydrous Copper (II) Chloride with Aluminum Powder.
3 CuCl2 + 2 Al --> 3 Cu + 2 AlCl3
As the reaction proceeded, a great deal of smoke / gas / fumes were given off. The interesting part of the reaction was the color of the
fumes given off. The fumes coming directly off the reaction looked to be a dark purple, which then changed to a yellowing color, and then turned to
white.
What is going on? What are these fumes and why are they changing color?
One of the products of the reaction is Aluminum Chloride which will sublime into a gas once formed. This might account for why there seems to be such
a large amount of fumes produced from a relatively small reaction and also one of the colors. What color is AlCl3 (g)?
As it floats away and mixes with the Oxygen in the air, could be reacting to form something else?
For those unfamiliar with this reaction, I have attached a picture I took which illustrates the different colors of the fumes,
http://i62.photobucket.com/albums/h104/mrjeffy321/CuCl2_Al/C...
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Huhm......colloidial copper possibly?
Tim
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I imagine the dense looking fumes are from AlCl3 (dimer in vapor phase) absorbing water vapor rapidly, similar to, but not nearly as dramatic as P2O5.
I saw an article about a purple vapor-phase complex of platinum chloride and aluminum chloride, but I highly doubt there is any platinum in there.
Maybe an analagous complex forms with copper???
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Wow, this looks interesting. You just mixed dry anhydrous CuCl2 and Al-powder? How did you light the mix?
The yellow color can be explained. That most likely is AlCl3, contaminated with small remains of CuCl2. Finely powdered CuCl2 is yellow/brown, so I
easily can imagine that a mix of AlCl3 and CuCl2 looks yellow.
I think the blue/purple color may be due to the high temperature of the smoke, which on cooling down becomes light yellow. I have done an experiment
my self with PbI2, which changes from deep red to yellow, when it cools down from 200 C or so to room temperature.
This experiment is something I really consider doing myself. Could you please provide some details on the amounts used, the mixing, how the mix is
ignited etc. ?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I`m wondering also if the water of crystalisation in the copper chloride will have any bad effect (like it not working).
and also if All parts are 100% dry, if a drop of water could trigger this reaction if used on a large enough pile?
and would it be considered a Thermit reaction even though it`s Chlorine standing in for what is Typically Oxygen?
Nice Pic MJ 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Actually the water might help the reaction along as it will react instantly with the AlCl<sub>3</sub> (or (AlCl<sub>3</sub> <sub>2</sub> <sub>2</sub> which heats up and heat helps speed things along which heats up and heat helps speed things along 
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I`ve just tried this reaction myself, using the dihydrate form of copper chloride and 10 micron alu powder.
roughly half a spatula of each.
it mixed well without incident.
and is quite easy to lite even with a simple match flame or lighter.
oddly I got a very Purple flame color from it also, I certainly didn`t expect that, the amount was too small to note the smoke color though.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
| Quote: | Originally posted by UnintentionalChaos
I imagine the dense looking fumes are from AlCl3 (dimer in vapor phase) absorbing water vapor rapidly
|
That is an interesting possibility I had not considered….the interaction with the water in the air changing the color of the fumes. It has been
quite humid and rainy here lately, so there is plenty of water in the air to absorb. I should try it on a dry day to compare the results.
| Quote: | Originally posted by woelen
I think the blue/purple color may be due to the high temperature of the smoke, which on cooling down becomes light yellow. I have done an experiment
my self with PbI2, which changes from deep red to yellow, when it cools down from 200 C or so to room temperature.
|
Another interesting possibility.
| Quote: | Originally posted by woelen
Wow, this looks interesting. You just mixed dry anhydrous CuCl2 and Al-powder? How did you light the mix?
[…]
This experiment is something I really consider doing myself. Could you please provide some details on the amounts used, the mixing, how the mix is
ignited etc. ?
|
For the reaction I preformed ‘on tape’, I mixed about 35.5 grams of anhydrous Copper (II) Chloride with about 4.7 grams of Aluminum powder (~200
mesh). To mix, I just stirred the two powders together and then poured the mixture out onto my reaction surface (which, in the picture, happened to
be a rock). The color of the resulting mixture was not that far off from the original color of the anhydrous CuCl2, just a bit gray-er. I ignited
the mixture using a small strip of Magnesium Ribbon (which was probably overkill).
The reaction lasted for about 30 seconds and produced a great deal of ‘smoke’. I was standing upwind of the fumes and was unable to detect any
odor coming off the reaction.
| Quote: | Originally posted by YT2095
I`m wondering also if the water of crystalisation in the copper chloride will have any bad effect (like it not working).
|
I first tried this reaction using CuCl2 * 2 H2O and did not get nearly as good results. Yes, the reaction did react, but it was more of a fizzling
reaction, not nearly as spectacular as when I used anhydrous CuCl2, also I did not observe the colored fumes coming off the dihydrate CuCl2.
But then you said you were able to get the reaction to easily ignite using the dihydrate, so maybe there was something wrong about my first try (using
the * 2 H2O), or is a particle size issue.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I think particle size played a part here, my blue Copper Chloride was almost white when I`de powdered it and 10 Micron alu is Very fine also.
I will also say that plenty metal (copper) does get atomised in the smoke too, I did mine in the Lab, and there`s a distinct copper type "Smell and
taste" lingering.
I can`t be sure, but I don`t think Non-Smokers can detect this as easily as a smoker can.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by YT2095
I can`t be sure, but I don`t think Non-Smokers can detect this as easily as a smoker can.
|
Why?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
copper and one or 2 other metals have a strange property that when a small amount is present in the mouth (even micro grams) they taste very sweet
when you take a drag off a cig.
I`ve no idea why it does this either?
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by mrjeffy321...
I first tried this reaction using CuCl2 * 2 H2O and did not get nearly as good results. Yes, the reaction did react, but it was more of a fizzling
reaction, not nearly as spectacular as when I used anhydrous CuCl2, also I did not observe the colored fumes coming off the dihydrate CuCl2.
But then you said you were able to get the reaction to easily ignite using the dihydrate, so maybe there was something wrong about my first try (using
the * 2 H2O), or is a particle size issue. |
Did you adjust the amount of aluminium to compensate for the water? While water takes a lot of energy to vapourise, it still reacts vigorously with
active metals as can be seen by spraying water on burning massive magnesium. But it is going to change ratios
2 Al + 3 CuCl2 => 2 AlCl3 + 3 Cu
4 Al + 3 CuCl2.2H2O => 2 AlCl3 + Al2O3 + 3 Cu + 6 H2
CuCl2 breaks down at high temperatures into Cl2 and CuCl, which forms complexes with chlorides even in the gas phase. AlCl3 will shed Cl2 at high
temperatures as well. So you could have a rather complex mixture of reactions, including AlCL3, free Cu, and CuCl reacting with air (O2 and H2O) as
they move away from the reaction.
Be interesting to stick bits of scrap ceramic tile into different regions of the smoke to see if you can trap reaction products, or if they continue
to react even if quick cooled.
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
One other suggestion for the purple colour:
Firstly, copper vapour is red in colour.
Secondly, copper(II) halides impart a blue-green colour to flames. Additionally, there may be a significant quantity of copper(I) chloride in the
reaction mixture, as a transitory product of reducing the copper(II) chloride. Copper(I) compounds impart a blue colour to flames.
A mixture of these colours will appear as purple.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
| Quote: | Originally posted by not_important
Did you adjust the amount of aluminium to compensate for the water?
4 Al + 3 CuCl2.2H2O => 2 AlCl3 + Al2O3 + 3 Cu + 6 H2
|
Yes, I adjusted for the increased mass of the Copper Chloride due to the water, however I just assumed that it would be the same chemical reaction
with the only difference being that the hydrated water within the CuCl2 would end up as steam in the end,
3 CuCl2 * 2H2O + 2 Al --> 3 Cu + 2 AlCl3 + H2O
But you suggest a very different reaction where the water reacts chemically with the Aluminum, thus significantly altering the ratios.
I just went out and tried a reaction using the ratios suggested by your chemical reaction.
I used a strip of Mg ribbon to ignite the mixture. The first time, when the Mg burned down to the powder, nothing happened except the powder and its
container seemed to get very hot and change color from the gray-ish blue color originally to a much darker gray color. Upon inspection, the powder
had hardened into a solid, brittle, lump. I decided to try it again using the left-overs of the first try. I stuck another piece of Mg ribbon into
the hard lump. This time when the Mg burned down something did happen. I saw a small blue flame appear (something I did not see with the anhydrous
CuCl2 reaction) and a lot of white fumes were produced (not much, if any, color). This time, the remaining material left over in the container was
white.
Overall, not a very spectacular reaction, but did give different results than when I assumed the water did not react with the Aluminum.
| Quote: | Originally posted by not_important
CuCl2 breaks down at high temperatures into Cl2 and CuCl, which forms complexes with chlorides even in the gas phase. AlCl3 will shed Cl2 at high
temperatures as well. So you could have a rather complex mixture of reactions, including AlCL3, free Cu, and CuCl reacting with air (O2 and H2O) as
they move away from the reaction.
|
So your saying it has to do with many of the substances (both reactants and products) decomposing due to the heat released during the reaction.
Chlorine gas would be a big product of the decomposition side-reactions occurring with both the Copper Chloride and the Aluminum Chloride potentially
breaking down and releasing Cl2. Chlorine gas is colored, but is not quite as opaque as some of the fumes seen on the picture. If significant
quantities of Cl2 are produced, it should be easy enough to detect the smell (not that I would want to).
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@I am a fish: I doubt that your explanation is correct. I agree with the flame colors, but then there should be light emission. If I look at the
pictures, then I think the color is due to absorption, instead of emission.
[Edited on 9-5-07 by woelen]
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
I do not recall seeing any color in the flame of the anhydrous CuCl2 reaction, just a bright yellow ball, not a blue flame, and the pictures/video I
have of the reaction seem to confirm this.
Also, the fumes seem rather thick and would not let much light transmit through them so as to mix with the color of any red Copper metal vapor, and
yet the purple fumes can sometimes extend farther away from the reaction than what is seen in the picture.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by mrjeffy321...
I just went out and tried a reaction using the ratios suggested by your chemical reaction.
I used a strip of Mg ribbon to ignite the mixture. The first time, when the Mg burned down to the powder, nothing happened except the powder and its
container seemed to get very hot and change color from the gray-ish blue color originally to a much darker gray color. Upon inspection, the powder
had hardened into a solid, brittle, lump. I decided to try it again using the left-overs of the first try. I stuck another piece of Mg ribbon into
the hard lump. This time when the Mg burned down something did happen. I saw a small blue flame appear (something I did not see with the anhydrous
CuCl2 reaction) and a lot of white fumes were produced (not much, if any, color). This time, the remaining material left over in the container was
white. |
Did you happen to dump the products into water to see if they'd dissolve, and what if anything was left?
Even without the extra aluminium the water will react with the aluminium chloride to give hydrated and basic chlorides.
| Quote: |
| Quote: | Originally posted by not_important
CuCl2 breaks down at high temperatures into Cl2 and CuCl, which forms complexes with chlorides even in the gas phase. AlCl3 will shed Cl2 at high
temperatures as well. So you could have a rather complex mixture of reactions, including AlCL3, free Cu, and CuCl reacting with air (O2 and H2O) as
they move away from the reaction.
|
So your saying it has to do with many of the substances (both reactants and products) decomposing due to the heat released during the reaction.
Chlorine gas would be a big product of the decomposition side-reactions occurring with both the Copper Chloride and the Aluminum Chloride potentially
breaking down and releasing Cl2. Chlorine gas is colored, but is not quite as opaque as some of the fumes seen on the picture. If significant
quantities of Cl2 are produced, it should be easy enough to detect the smell (not that I would want to). |
I'd expect a rather complex mixture being formed, much different than standard thermite where iron and Al2O3 are by far the majority products.
Chlorine would tend to quickly recombine, Al(I)Cl is going to react when it drops below a 1000 C or thereabouts, CuCl a bit lower. On top of that any
copper metal vapourised is going to react with air, Cl2, or CuCl2, to give oxides, oxychlorides, and CuCl.
I'd expect the more strongly coloured portion to be due to Cu(I) chloride and/or oxide, possibly with some free Cu metal, possibly from Cu(I)-Cu(II)
mixed oxidation state compounds. As woelen said, the high temperature may change the colour of the compounds, if you've test tubes to spare you could
try sacrificing one by putting a little CuCl2 in it and running it up to dull red heat.
As the temperature drops and the AlCl3 reacts with moisture, the liberated HCl and oxygen react with the copper compounds. Note that copper complexes
with Cl(1-) to give green-yellow-brown colours.
Attempting to capture some of the smoke, either on tile or in a container, could confirm the presence of copper and possibly its oxidation state.
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
Yes, I did dump the products into water when finished, although I cannot say for certain whether it all dissolved or not. I think it did, but I am
not sure.
What I can do is duplicate the reaction, but this time do it inside of a makeshift, stainless steel, crucible with a loose fitting cap. As the
reaction proceeds, the fumes evolved will be temporarily trapped inside and be able to mix with each other and cool on the inside of the container.
Then we will be able to see what color vapor finally emerges and what gets left behind inside the container.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did the reaction a few times on a small scale (200 mg of anhydrous CuCl2 and 30 mg of Al, 45 um spherical powder, this ratio is according to 3CuCl2
+ 2Al --> 3Cu + 2AlCl3). I used reagent grade chemicals to assure that the colors are not due to impurities.
The mix was fairly easy to ignite, although not with a simple cigarette lighter. A propane torch was needed, but once the mix was going (burning with
a bluish flame) it burned brilliantly. This reaction indeed is amazing, and beautifully colored smoke is produced. I put the tip of an old screw
driver just above/in the flame and in this way, I could capture some of the smoke. I also collected some of the solid remains after the reaction.
The tip of the screw driver is covered with beautifully colored remains, with purplish red colors, white and yellow colors.
Here follows the result of one capture experiment, with red/purple material and white material on the tip of the screw driver:
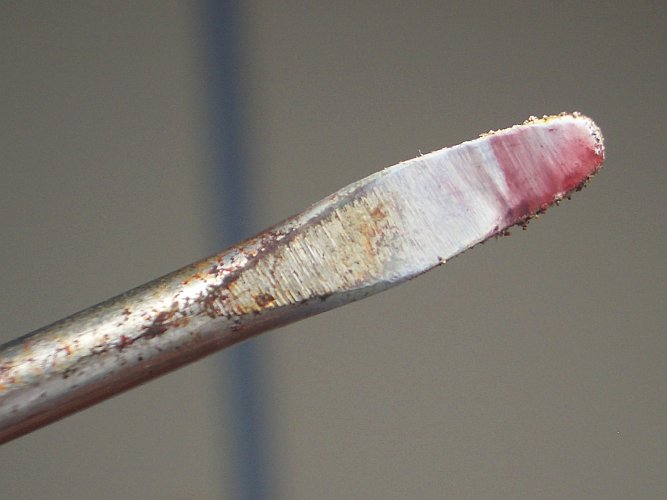
A second attempt gave the following result:

I scraped off some of the reddish material. This does not dissolve in water. It makes the water turbid and grey/brown. The amount is too low to be
fully conclusive, but I think it is very finely divided copper metal.
When the red material is left on the screw driver, and it is breathed against (humid air), then its color does not change.
The remaining ashes are grey/black. When this is added to water, then a turbid white liquid is obtained, with some brown/grey solid at the bottom.
When some hydrochloric acid is added, then the liquid becomes almost clear, and the brown/grey material at the bottom partly dissolves and the color
intensifies. It becomes more red/brown. The red/brown material does not dissolve, not even after 1 hour. So, I am quite sure this is copper metal.
The following picture shows the contents of the test tube, photographed from below:
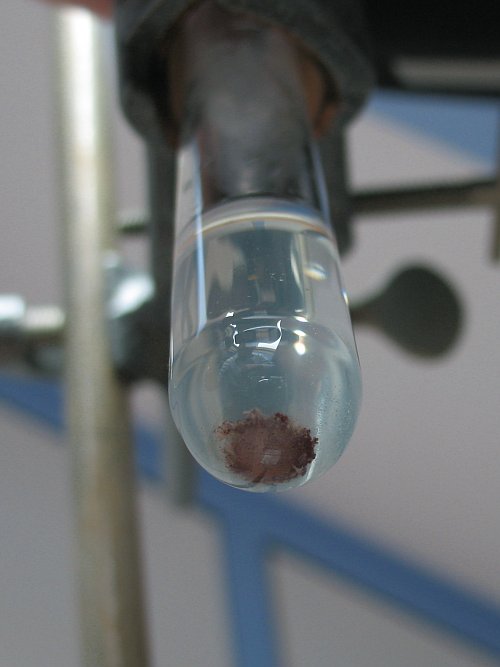
[Edited on 18-11-11 by woelen]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Sure looks like the copper reds of ceramic glazes which are Cu, Cu2O, or a solid solution of Cu2O in Cu depending one who you believe. I'd expect Cu
with some dissolved oxygen (as Cu2O), if there's not too much oxygen the mixture will act just about like the metal.
Going by the rest of your results I suspect that the white is a mixture of hydrated aluminium oxide and chloride, which should result in an acid
solution in DW. The hydrated oxides will not dissolve in plain water, but adding HCl would dissolve them. The yellow colour may come from Cu salts,
adding strong aqueous ammonia to the clear solution might tell as even a small amount of copper ions will give a blue tint.
Nice bit of work there, the second attempt captured just about everything.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Looks interesting! If the reaction is done in a dry test tube, you should be able to get a sublimate of anhydrous AlCl3.
Other thermite reactions with anhydrous metal chlorides might also be useful for synthesis of AlCl3.
AlCl3 tends to form HCl and basic chlorides or even the oxide with aerial moisture, so that AlCl3 which has pulled moisture from air does only
partially dissolve in water.
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
I just performed the reaction (total of 15 grams of mixture) inside of a semi-sealed stainless steel container which was immersed in very cold, wet
sand.
I lit the end of a Mg ribbon fuse, placed the loose-fitting cap onto the container and stepped back to watch the results.
As the reaction proceeded, the only color smoke I saw emerge from the container was white.
After the reaction was done, I waited a few minutes for the set-up to cool (it was very hot to the touch immediately afterward).
Around the outside edge of the opening of the container I found a few traces of a white, powdery deposit, but not very much. On the inside, the
container was completely coated with a reddish colored substance which looks a whole lot like my supply of Copper (I) Oxide, Cu2O.
When I poured some water into the container, immediately the container got very hot and some whiteish fumes were given off. The resulting mixture was
the same color as the previously dry left-over powder covering the inside of the container.
It is a little hard to tell right now, but I don’t think a lot of the red remnants dissolved in the water, I think they just formed a suspension and
will eventually settle down to the bottom (and a good portion already has done so as I have been typing this).
Here is a picture of what the inside of the container looked like before I added water,
http://i62.photobucket.com/albums/h104/mrjeffy321/CuCl2_Al/I...
So it looks like the white smoke is due to all of the Aluminum Chloride subliming away. The colored smoke (purple and yellow) would be due to the
Copper compounds which can be collected and removed from the vapors given off fairly easily.
|
|
|
mrjeffy321
Hazard to Others
  
Posts: 149
Registered: 11-6-2005
Member Is Offline
|
|
I have performed a similar reaction using Zinc powder in place of the Aluminum powder.
The Zinc powder, I think, is of a much finer particle size than the Aluminum powder I was originally using.
Mixing the Zinc and CuCl2 together is not as easy as it was when using Aluminum.
Simply stirring the two powders together causes the mixture to get very hot (hot enough to melt the bottom of a thin plastic bowl). I have not seen
it auto-ignite, but merely mixing the powders together at room temperature does liberate a good amount of heat.
The reaction is very easy to ignite. I was easily able to ignite the mixture with the flame of a butane torch, but I also was successful in igniting
it with a single drop of water.
The Zinc reaction is not quite as violent as when using Aluminum, but it does proceed quickly. No colored fumes are given off when using Zinc, as is
the case when using Aluminum, just lots of white fumes.
Whereas the Aluminum reaction left a darker colored solid byproduct behind, the Zinc reaction leaves an off-white colored by product behind which
seems to dissolve/react exothermically when was is poured on it.
EDIT:
I tried it again using Aluminum.
I was not successful in igniting the mixture using a drop of water, nor was I successful when using a drop of NaOH or HCl solution.
My guess is that this is due to the particle size of the reducing agent using in the reaction.
When I dropped the water onto the mixture, in the spot where it landed, it did heat up a great deal, so much so that it boiled away the water (or most
of it), but it did not begin the reaction nor did I see any colored fumes.
I found that just mixing the Aluminum powder and the CuCl2 does indeed generate some heat, but not nearly as much as when using Zinc.
[Edited on 5-15-2007 by mrjeffy321]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This heating up is quite common. If you have AgNO3, then try mixing a fine powder of it with some powdered Zn or Mg and add a drop of water (using a
LONG stick, and using 200 mg or so at most). A white flash will be produced and droplets of molten silver are sprayed around!
Copper(II) is less reactive than silver(I), but still it can produce a lot of heat. So, I am not surprised by the results you give here. Any soluble
oxidizing metal salt (salts of silver, copper, mercury, gold) and strongly reducing metal will show this reaction, when a small amount of water is
added.
|
|
|
sienchoon
Harmless

Posts: 2
Registered: 17-11-2011
Location: Kuala Lumpur
Member Is Offline
Mood: Confused
|
|
Why?
Hmmm... I had this similar experiment in the lab a few days ago. I had 0.1M of Copper (II) Chloride solution added with 0.1g of aluminum powder. (i
know its quite a small amount) but there wasn't a significant reaction. All i could see is some sediments at the bottom of the test tube and some
precipitate on top.. its like they were seperated. the colour was still light-bluish but a little grey... i'm not sure why the reaction was not
vigorous as it should be as mentioned in the textbook. Does this have to do with the Galvanic Metal series? :/ someone help me pls
|
|
|
| Pages:
1
2 |