Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Platinized silica wool catalyst from e-cigarette wick
Abstract
I successfully preparared platinized silica wool from silica wick used in e-cigarettes. The wool was able to catalyst the oxidation of propane and
ammonia in air.
Introduction
I am planning on building an Ostwald reactor to oxidize ammonia into nitrogen oxides and then subsequently dissolve them in water to produce nitric
acid. One key part of the reactor is the catalyst on which the oxidation happens. I saw that chemetix had success with his reactor without the use of
platinum based catalyst (http://www.sciencemadness.org/talk/viewthread.php?tid=71282&...). I know most people want to avoid the use of platinum because it is so expensive,
but since I needed it for other projects (and element collection) anyway, I went ahead and bought 10 grams of platinum (for about 350 $).
Having nitric and hydrochloric acid, preparing hexachloroplatinates and reducing them to fine metal particles was not a problem (I'll outline the
procedure below for reference). What was more difficult to find however was a support for the catalyst. The support has to be resistant to nitric acid
and nitrogen oxides, resistant to the extreme heat during the reaction (which might exceeds 1000 °C, depending on a number of parameters of the
reactor) and have a sufficient surface area for effective reaction.
Astral Chemistry (on youtube: https://www.youtube.com/watch?v=iaNSH89gpPk and https://www.youtube.com/watch?v=dMV4-CxCyL0) prepared platinized quartz wool and successfully used it in an Ostwald reactor, but I couldn't find
quartz/silica wool for reasonable prices (100 + $).
After a bit of looking around I found out that e-cigarette use a wick to vaporize the propylene glycol e-liquid and that the wick is usually made of
pure silica. So I bought 1 meter of 3 mm wick for 3 $ and experimented a bit.
Experimental
Melting test The first thing I tried was putting the wick in a propane flame to melt it. Even with my huge propane torch (not
your standard disposable canister type torch) I did not manage to melt any of the wick. So the heat resistance part is covered. Since the wick is
silica, the acid resistance part is likely covered too, bit I haven't tested it directly.
Preparation of the catalyst The wick is constituted of 5 strands in turn consisting of very fine silica fibers, much finer than a
human hair. I untwisted the strands and tried to spread the fibers as much as possible to maximize the surface area of the silica. This was done under
water to preved fine silica fibers from going everyrwhere. The silica wool thus obtained was plunged in a solution of potassium hexachloroplatinate (a
few miligrams, leftover on a filter paper from the preparation) in about 15 ml of water. To this I added hydrazine sulfate (1 gram) and sodium acetate
(1 gram) solution in 15 ml of water. After 1 hour the solution appeared unchanged, so I added a few mg of sodium hydroxide and placed the solution in
a hot water bath. After 15 minutes, the solution had lost its color and platinum particles had deposited on the silica. I strained the silica wool,
rinsed it with water and dried it on a flame.
Test of the catalyst The resulting platinized wool was quite brittle but the fibers held together more or less. To test the
catalytic activity, I heated the catalyst with a propane flame, extinguished the flame and placed the catalyst in the gas flow. After a few seconds,
the catalyst started glowing red indicating that there was some platinum on the surface. I also did the same test with ammonia vapor, but oxidation
was more difficult to sustain although it did work for several seconds. I think a proper mixture air to ammonia is necessary for sustained oxidation.
Preparation of K2PtCl6
For reference, here is how I prepared the potassium hexachloroplatinnate (based on Brauer's method)
A platinum metal piece (0.59 g) was added to a mixuture of 32 % hydrochloric acid (7.5 ml) and 65 % nitric acid (2.5 ml). The mixture was heated near
boiling for a few hours. After volume had been reducted to about 5 ml, I added hydrochloric acid (10 ml) and boiled down to 5 ml again. This was
repeated once more. At the end no brown fumes were escaping the solution. The volume was reduced to 1 or 2 ml and potassium chloride (3.72 g, 3:1 mass
ratio to hexachloroplatinic acid) was added in one portion with stirring. The canari yellow precipitated that resulted was filtered, rinsed with
deionized water three times, then with acetone once and dried at 50 °C for an hour.
Final yield: 1.30 g (88 % based on platinum)
I don't know how long the catalyst will last however. Maybe it is easily poisoned, or the platinum can evaporated at high temperatures?
The main problem with those silica wicks is that they seem to be made of rather short fibers, which are dispersed in the air when you manipulate them.
For this reason I separated the strands under water. In fact the water is full of silica fibers when you are done separating the wick. The fibers stay
together enough to work with, but you can't manipulate the catalyst too much other wise it falls apart. This should not be an issue when the catalyst
is secured in the Ostwald reactor tube.
Update coming when I have finished building the reactor.
Legends left to right, top to bottom:
1. Platinum bar
2. Hammered platinum bar to make it thinner (future platinum electrode for electrolysis)
3. Silica wick 3 mm diameter
4. Potassium hexachloroplatinate
5. The finished catalyst attached to steel wire. Slightly gray color from platinum
    
[Edited on 1-7-2018 by Heptylene]
[Edited on 1-7-2018 by Heptylene]
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
To have a 'play' I bought a catalytic hand warmer and a couple of spare catalytic pads https://www.ebay.co.uk/itm/1pc-Pocket-Heater-Hand-Warmer-acc...
Based on the price with p&p being 99p each I did not expect much.
The hand warmer works very well... an overall red glow.
So,
. it may be worth stuffing a few in a tube as a test as they withstand red heat
. an extremely small quantity of platinum is required.
Have you considered new or used automotive catalytic converters ?
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I don't really have any source of catalytic converters from old cars. Besides I think those might be difficult to use because they have a fixed size
and are designed for flowrates from a car exhaust (Hundreds of liters per minute/LPM of gas I'm guessing). For reference my lab vacuum pump puts out
20 LPM, and my shitty aquarium air pump puts out about 2 LPM. Absorbing large flowratesof nitrogen oxides could be difficult. Those catalytic
converter might also be hard to heat from the outside.
I'll stick to making my own catalyst for not (especially since I now have all the materials needed!)
Those catalytic lighter pads seem very interesting! By far the cheapest source of high surface area platinum catalyst I've seen. Making your own
catalyst as I did has the drawback that you have to buy some platinum (1 gram Pt bar minimum) to get started, so that's about 50 $ initial cost. Even
though only miligrams of platinum are actually present in the catalyst.
I'm intereseted in the result you get with those catalytic pads!
Btw I did a quick test of reaction inside a glass pipette stuffed with the catalyst I just made. I pumped ammonia vapour and air and heated the
catalyst with a torch. When I removed the flame the oxidation continued for about a minute before the catalyst cooled down too much. (see image). The
tube also melted from heating with a torch. I'll use quartz tubes next time.

[Edited on 1-7-2018 by Heptylene]
[Edited on 1-7-2018 by Heptylene]
|
|
|
walruslover69
Hazard to Others
  
Posts: 216
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
How do you plan on actually constructing the reactor?
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
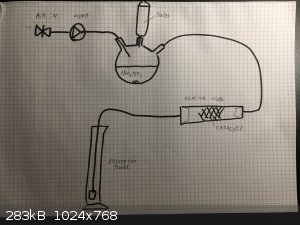
walruslover69 (nice username hehe), the design I imagine right now is in three parts:
1. Ammonia generator: 3-neck RBF containing ammonium nitrate solution and a strong stirbar. Addition funnel containing sodium hydroxide solution
fitted to the central neck. Another neck will be an air inlet, and the remaining neck will be the ammonia + air mixutre outlet.
For the air supply I'll use the air outlet of my vaccum pump with a needle valve on the vaccum side, this way I'll be able to fine tune the air
flowrate (0 to 20 LPM). By carefully controlling the rate of addition of NaOH, the ammonia gas flowrate can be controlled.
2. Reactor tube: Fused silica tube (16 mm diameter, 1 mm thick walls) containing some catalyst I made in the middle. This will be heated at the start
with a blowtorch and hopefully the reaction will be self sustaining. If not I'll use some candles (the flat type used for stoves etc.) to keep the
tube warm, or at some point build a heating element from nichrome wire.
2.5: suckback trap between reactor tube and absorption tower.
3. Absorption tower: Probably just a large beaker full of water with a tube leading into it. I might use Na2CO3 or NaOH to absorb the gases and make
sodium nitrate and nitrite instead of nitric acid.
The motivation behind this reactor (besides the fun) is that I have 2 kg of impure NH4NO3 fertilizer that I want to convert to NaNO3. Adding NaOH to
this would release about 600 liters of ammonia gas. Since I need some way to dispose of the gas, I might as well convert it to more nitrates!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
For the ammonia reactor, I believe a mixture of solids in the RBF would work better than two solutions. Slowly dripping water from the addition funnel
leads to release of ammonia. It's so soluble in water, having two solutions will suck up much of the produced ammonia. (Until they get saturated, I
guess.)
|
|
|
Texium
|
Thread Split
7-7-2018 at 07:29 |
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Could you use kaowool instead of the silica/quarts thread? I'm guessing that it would hold up to the temp with no problem but IDK how the platinum
would attach to the structure.
Another idea to get more surface area would be to shred the silica wick into it's fine pieces in the platinum solution, then filter the solution and
collect all the fine pieces. Then use Kaowool to pack your quartz tube (exit side) and then stuff the fine pieces on the entry side. You may need a
little kaowool to hold it in place, in front of the catalyst and or you could use non-coated wick on either side.
On a side note, now is a good time to buy platinum or palladium as the prices are just about a low as I've seen in many years.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
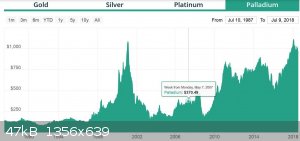
Not according to the kitco historical charts for palladium!
Hm. That doesn't show up very well on the thumbnail. But go to kitco and look up the 5 year historical palladium chart to find it. 10 years ago, it
was only $200/oz!
On a side note, palladium is one of the last two elements I need for my collection. That and rubidium.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by MrHomeScientist  | Not according to the kitco historical charts for palladium!
Hm. That doesn't show up very well on the thumbnail. But go to kitco and look up the 5 year historical palladium chart to find it. 10 years ago, it
was only $200/oz!
On a side note, palladium is one of the last two elements I need for my collection. That and rubidium. |
Wow, you are right. I swear I had looked at Pd 2-3 years ago and it was close to $1,500-1,600, guess not. As for Pt, it was almost $2200 a little
over 12 years ago, so in comparison the price now is pretty reasonable.
Here is the historical pricing for 4 precious metals, there seems to be a trend. I think I mixed up Palladium and Rhodium in historical pricing as it
had been $25-2600!
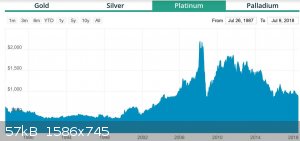
[Edited on 7-9-2018 by RogueRose]
[Edited on 7-9-2018 by RogueRose]
[Edited on 7-9-2018 by RogueRose]
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | | For the ammonia reactor, I believe a mixture of solids in the RBF would work better than two solutions. Slowly dripping water from the addition funnel
leads to release of ammonia. It's so soluble in water, having two solutions will suck up much of the produced ammonia. (Until they get saturated, I
guess.) |
I just tried mixing fairly concentrated (but not saturated) solutions of NaOH and NH4NO3, and indeed there is almost no gas evolved. With solid NH4NO3
and adding 50 % NaOH solution, the gas evolution is much better. I think mixing all the solids and adding water could lead to a runaway reaction which
I don't want. I'll do some more testing and a pilot run soon, I just received the fused silica tubes for the actual reactor. I couldn't melt those if
I tried!
I'm thinking I should start a new thread for the rest of the reactor design, this one was mostly about the catalyst support. I'll post a link here if
I do.
@RogueRose: Kaowool might work too, as long as it doesn't melt. Apparently different grades exist that withstand different temperatures. To get the
platinum onto it, the same method I used for silica would probably work too. Just reduce the hexachloroplatinate(IV) ions to Pt metal in solution and
hope some adheres to the surface. Astral chemistry used ascrobic acid, I used hydrazine. Then wash w/ water, dry and put the catalyst in a blowtorch
flame to sinter the particles.
I like the idea of shredding the silica wick and holding it with something else. If my catalyst is not active enough I might try that next.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
I did the reaction of solid KOH and NH4NO3 adding a bit of water and it did indeed heat up a lot causing a lot of the water to boil.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I took some of the fused silica wick (which had some burnt nicotine/e-cig juice on it) and heated with a blow torch to clean it. I did this with a
number of pieces each about 1cm long. They looked pretty clean afterwards, whitish grey. I was able to get them into a ball/pile and it increased
the exposed surface area greatly.
I think another source for this quartz, if small quantities are needed, is inside some nichrome heating coils. I've taken apart many heat guns and
they have a fibrous material that is very similar to this in texture.
I think I missed the part where you heated the silica w/ platinum on it to sinter it, well I guess I didn't think about that happening when you heated
it. That makes sense as to why it would stick then. I thought maybe something in the solution helped make the Pt stick to the silica.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Maybe I wasn't clear when I said I dried the catalyst in a flame. That treatment is to remove water and is likely mandatory to bind the Pt particles
to the substrate, although I haven't tested without. Btw new thread on the reactor itself here
I don't know what those heating element sleeves are made of, but its likely silica/quartz as it has to hold up to the temperature. Maybe mica would be
useful to make structural parts for a catalyst, or even as substrat itself.
|
|
|