| Pages:
1
2 |
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Catching FOX no. 7 (with pics)
1,1-Diamino-2,2-dinitroethene or FOX-7 is an explosive developed in the late 1990's by the Swedish Defence Research Agency. FOX-7 shows significant
improvements in sensitivity compared to RDX, particularly in response to friction and shock.
Molecular formula: C2H4N404
VOD : 8870m/s
Density : 1.88g/cm3
O2 balance : -21.6%
Molar mass : 148.08
I will be using FOI method 2 taken from the pdf file "FOX-7 a new insensitive explosive" by DSTO group.
All reagents were purchased or synthesized to as high standard as possible.
The synthesis is split up into three steps, the following is the first step performed in a half size batch.
REAGENTS
* 430ml methanol (dried with MgSO4 and freshly distilled)
* 116ml 30% Sodium methoxide solution (made with Na metal)
* 18.24g Acetamidine hydrochloride (lab grade)
* 27.92g Diethyl oxalate (collected at still head temp 170-185)
* 200ml dry methanol to dissolve Diethyl oxalate in
2-METHOXY-2-METHYL-4,5-IMIDAZOLIDINEDIONE
A 1 litre three neck flask was charged with 430ml methanol, and 116 ml 30%wt sodium methoxide solution.
18.24g Acetamidine hydrochloride was added and the solution stirred. The flask was fitted with a dropping funnel, drying tube and the final neck was
just stoppered. Solution is milky white.
The diethyl oxalate in methanol was added at a slow drip rate over a period of 1.5-1.7 hours and then the reaction mixture was stirred for an
additional hour. Solution has changed to light yellow after addition.
The whole reaction mix was dumped into a 2 litre beaker and acidified to ph 4 by the addition of 35% concentrated hydrochloric acid and ph test
strips. Temperature did not rise much at all during this step.
The solution was double filtered with a vacuum pump and buchner and the solution (now clear) placed in a pyrex oven dish to evaporate. The paper says
the temperature of evaporation must not be above 30C which means nearly 750ml of methanol plus a small amount of water must be evaporated at room
temperature  . .
Will update upon recovery of the solid product.
 



[Edited on 6-7-2018 by greenlight]
[Edited on 6-7-2018 by greenlight]
[Edited on 7-7-2018 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Following this closely, as FOX-7 is something i've always been interested in.
Just one thing, i'm not sure what the actual reaction you are doing here, why are you making the imidazolidinedione? Is it a precursor to
2-methylimidazole? Just confused why that is starting point in the reaction diagram but not mentioned. I am slow though so sorry if its obvious
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
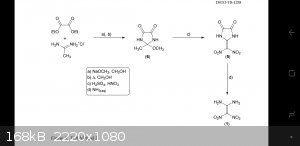
I accidentally uploaded the wrong diagram haha.
The 2-Methoxy-2-methylimidazoledinedione is the heterocyclic intermediate product. Nitration of this intermediate and then hydrolysation with ammonia
produces the final FOX-7.
[Edited on 6-7-2018 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
The need for FOX-7 in cast-cured formulation has been eliminated by various reduced sesitivity RDX formulation.
FOX-7 is still going to beat RDX in pressed formulations, though. Seeing a pressed formulation: 50 % FOX-7, 10 % AP, 10 % polymer and 30 % Al would be
nice.
|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
Sounds fun, if I could find a route to the reagents I would love to try it.
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
2-METHOXY-2-METHYL-4,5-IMIDAZOLIDINEDIONE is one of them.
Oh, hello!  |
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
Yeah, I know. I would need to find routes to work towards the starting materials.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
The biggest problem with this method both on an amateur scale and a commercial level is the first 2-methoxy-2-methyl-4,5-imidazolidinedione step.
The reagents are hard for an amateur to find but the rest of the synthesis just requires easy to get acids and ammonia solution.
The other problem is the amount of solvent used. In the paper, the author uses over 1.5 litres of methanol to yield just under 20 grams of FOX-7.
Using less solvent significantly lowers yields so on an industrial scale the reactors would have to be huge and not to mention the quantities of dry
methanol needed.
On a brighter note all 750ml of methanol has nearly evaporated and the solid has crystallized out on top of the small amount of water remaining from
the HCl addition. Soon I can recrystallize to get rid of the sodium chloride impurities before nitration.
[Edited on 9-7-2018 by greenlight]

[Edited on 9-7-2018 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
why not bubble dry hcl gas in vigorously stirred mixture? then no added water!
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes, that is actually one of the alternative ways outlined in the paper. I just did it with the aqueous solution because I was pressed for time.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Acetamidine HCl
http://www.orgsyn.org/demo.aspx?prep=CV1P0005
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I would think that with aspirator vacuum and a nice tight setup, it would be possible to distil the methanol below 30 C. Then you could recycle the
solvent.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
This is good suggestion and if I repeat the synthesis, I will try it.
It seems methanol boils at 26.94'C at 140 mm/Hg from a quick Google search.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Seems like a good application of a short path condenser too. Mild vacuum would help, but keep pumping freezing water though the condenser and you'd be
able to recover a lot of the methanol.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Ooh I don't have a short path condenser yet, must put it on the list.
I just finished drying the recrystallized product. There is an unusual error in the paper where it says the solid was dissolved in 320ml methanol,
insoluble salts removed, and then the volume was reduced to 320ml?!?! before placing in a refrigerator.
Well I dissolved my 28g of solid in 160ml of boiling methanol because it's a half size batch and stirred for a few minutes, hot filtered, and the
reduced it to about 1/3 of the initial volume. Then I placed it in a freezer overnight and filtered the next day.
I have 9-10g of insoluble crap and 14g of the intermediate product. This corresponds to only a 53% yield compared to the papers 64%
I have lost about 4 or 5 grams, but nevertheless, I will perform the nitration and alter the acid amounts accordingly to the loss of product.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
53% on the first run compared to the paper's 64% is mighty fine work and nothing to be sad about I think, great work so far
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thanks Tdep
Looking over the next step it involves filtering a concentrated mixture of acids at the end with no water dilution like most He's. That shit eats
straight through my filter papers,I just did a test with mixed acids.
I am going to have to order a better filtering funnel, maybe fritted glass for the next step before I proceed.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Trick is get some fiber glass insulation wash thoroughly then blend it a few times fast, then as a wet slurry deposit it on your filter paper a few
times to build up thickness,, then let dry, care fully separate it when damp then allow to dry fully.
It is good for one shot, I use this method to filter concentrated sulfuric acid in a large funnel.
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I assume that's only good if you're interested in keeping the filtrate. I'm not up with the FOX-7 procedure but I suppose you want to collect the
precipitate and remove it from the mixed acids, in which case a glass frit is good. I have used one many times and I stand by them. Just maybe don't
pull those fumes into a pump you like.... pulling them into a pump you hate is fine though
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes, I need to separate and keep the precipitate which is highly acidic and is one of the worst things to filter. I will put a glass fritted funnel
on order.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Quick update on the FOX-7.
While waiting for my glass funnel, I had an "it will be fine, I will separate it" moment and performed the nitration anyway. It ran perfectly
smoothly and due to having no proper filter I crudely tried to scoop out the fine light yellow crystalline product.
I managed to get most of it out and lay it out to dry but it was still too wet and by the next day the residual sulphuric acid had sucked so much
moisture out of the air it was a wet mess and there was barely any crystals left.
I have just written to say I have more oxalic acid on the way to make fresh diethyl oxalate and I will be running it again from the start as soon as I
can, so no I have not given up on this synthesis just taken a few steps back.
Just to make it worse my funnel arrived the next day
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I have just re-run the first step but this time I bubbled dry HCl gas through the stirred solution to acidify it. No pesky water this time
Now to wait for the methanol to evaporate again.
[Edited on 17-8-2018 by greenlight]
[Edited on 17-8-2018 by greenlight]

The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Finally had time to do the nitration of the 2-methoxy-2-methyl-4,5-imidazolidinedione. This time I yielded 14,95 grams which equates to about 55.5%
yield, slightly more than last time. I recalculated the amounts of acids for my amount of intermediate product.
REAGENTS
* 14.95g 2-methoxy-2-methyl-4,5-imidazolidinedione
* 83.66ml 98% Sulfuric acid
* 18.11ml 70% Nitric acid
2-2-DINITROMETHYLENE-4,5-IMIDAZOLIDINEDIONE
The 1 litre 3 neck flask was fitted with a dropping funnel, CaCl2 filled drying tube and thermometer and placed in an ice bath on top of a hotplate
with stirrer.
83.66ml of 98% sulfuric acid was added and chilled down to about 10 degrees C. The 2-methoxy-2-methyl-4,5-imidazolidinedione was added in small
portions with the stirrer on. No temperature rise was noted during the additions. Everything dissolved and formed a clear but yellow solution.
The dropping funnel was filled with 18.11ml 70% nitric acid and the addition was started with the stirred solution at 5 degrees C. After the first
few additions dropwise, the colour changed to a dark red wine texture. I never let the temperature rise above 12 degrees just to be safe (paper
states 30C). When almost all the nitric acid was added, the mixture all of a sudden went hot pick and crytals precipitated almost instantly. Quite a
pretty sight The colour quickly faded to orange and then beige as the last of
the acid was added. The colour quickly faded to orange and then beige as the last of
the acid was added.
The flask was taken out of the ice bath and stirred at room temperature for 30 minutes before filtering through a fritted filter this time. I still
lost a decent amount of product as it is so fine that a lot of it passed through the frit. What was collected is currently drying further in a
desiccator over anhydrous calcium chloride. I am not sure this is going to work but I do not want to risk it turning to a puddle drying in the open
because it is damp with hygroscopic acid. I wish there was a solvent it could be washed with before drying it.
[Edited on 5-9-2018 by greenlight]
[Edited on 5-9-2018 by greenlight]
    
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Cool photos. Could you have maybe washed it with some chilled azeotropic nitric or red fuming nitric acid? Never a perfect solvent but it might go a
way to removing some of the sulfuric
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
On the paper it just says air dried but I am sure it will just get even wetter in air. I have it in the desiccator and it has not dried one bit more.
You might be on to something there about washing with nitric. I think I will just run the final step with it damp because it is getting put into
water anyway.
This stuff takes the cake for most annoying to filter and dry. Imagine making PETN for example and not dumping it into water before filtering but
instead trying to filter and process it straight from the nitration bath Not
fun Not
fun
[Edited on 5-9-2018 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
| Pages:
1
2 |