| Pages:
1
..
6
7
8
9
10
..
17 |
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by Antwain
Sorry to post a half-informed post, but I just read the last couple of pages, and haven't looked at the patent... but it occurs to me that the
reaction at the anode may be reducing the permanganate? I could just be plain wrong about that, but has anyone tried separating the anode and cathode
with a salt-bridge?
|
Yeah! You are just plain wrong!... 
If you HAD read the previous posts and the Japanese patent, you would realise that reduction at the CATHODE is apparently not a big issue. The
japanese boffins don't even seem to bother about it in their lab scale experiments. Industrial electrolytic oxidizers use short stubby cathodes with
very high current/surface area, this inhibits reduction. In the older style electrolytic cells, porous material was wrapped around the cathode! In my
first, ridiculous, half-hearted attempt at oxidising alkaline MnO2 I used a separate anode/cathode chamber (plantpot), (see my first post up the
thread).
In my later cells I have used a short stubby cathode (the iron rod) it only dips about 10 mm into the electrolyte.
I'm not saying reduction doesn't occur, but we are trying to emulate the Japanese patent, and they don't even mention it (from memory). I suppose,
that on a very small scale (< 1 litre) it could become important, but at this stage, unless someone can come up with some convincing evidence, I'm
not prepared to build a double cell, what with all that near boiling NaOH, etc....
Regards, Xenoid
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
It was my understanding from reading a book on industrial electrochemistry (from the 1950's, and speaking from memory as I don't have it with me) that
commercial permanganate production involved making sodium permanganate by electrolysis of a sodium manganate solution, and the producing the potassium
salt by metathesis with KCl, all due to solubility issues.
I'll recheck the book when I find it.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by Eclectic
It was my understanding from reading a book on industrial electrochemistry (from the 1950's, and speaking from memory as I don't have it with me) that
commercial permanganate production involved making sodium permanganate by electrolysis of a sodium manganate solution, and the producing the potassium
salt by metathesis with KCl, all due to solubility issues.
I'll recheck the book when I find it. |
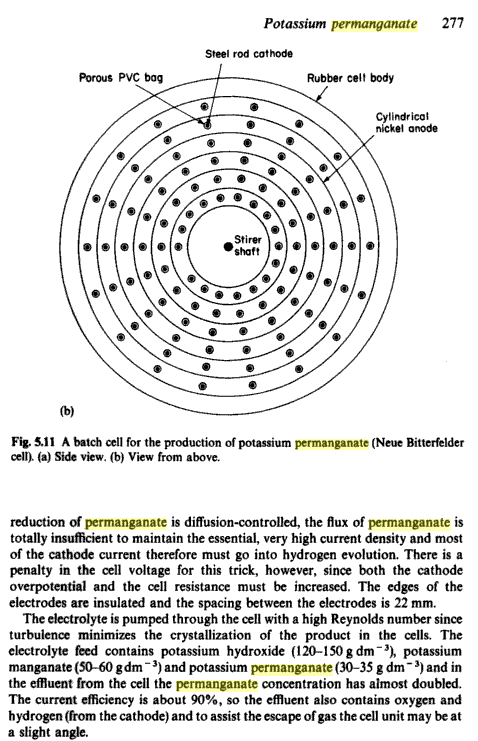
Most modern cells, electrolyse K-manganate to K-permanganate, the cells are specially designed to have high Reynolds numbers (I assume that means high
turbulence) to inhibit crystallisation of the poorly soluble K-permanganate.
What I am trying to achieve is basically what you have stated above, but only because I have plenty of cheap solid NaOH, but no solid KOH for the
fusion process.
Regards, Xenoid
[Edited on 15-9-2007 by Xenoid]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Wouldn't high turbulence tend to cause excessive nucleation and a dispersion of KMnO4 rather than crystals?
Tim
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by 12AX7
Wouldn't high turbulence tend to cause excessive nucleation and a dispersion of KMnO4 rather than crystals?
Tim |
I guess the high turbulence is to stop crystallisation building up on the anodes and other parts of the system ie. cathode separators, pipe work, etc.
and "clogging up" the cell, with presumably disasterous consequences.
Regards, Xenoid
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Turbulence
I got the impression the turbulence was to control deposites other than crystallization. "Oxidic deposites" I think was the term used in a patent I
read. MnIII and MnIV I guess. The mother liquor spend a lot of its time a cruddy mess.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
From "Industrial Electrochemistry"
Here's where I got my information about turbulence!
Sorry it's only a screen dump from Google Books!
Regards, Xenoid
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Fascinating. I had no idea that violent movement would inhibit crystallization. That must make the operation of a crystallization tank rather simple.
The decrease in velocity on entry into the larger volume would seem to do it. It seem these amateur cells will be better with somewhat frantic
stirring until harvest time.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Well I've just completed my latest attempt at making K-permanganate.
The cell is the same as last time except I have made a new lid from 15 mm. thick, grey, PVC sheet which can withstand the high temperature and
oxidising conditions better. I also added a 100 mm. long, 6 mm. diam. SS tube to the vent hole, to stop the clear PVC vent hose softening and
distorting. Initially I started the cell operating from a 5 Volt, constant voltage computer SMPS. The cell was running at about 7.5 - 8 Amps for about
3 hours but I found it was impossible to maintain a constant temperature, there was a lot of thermal runaway. Since I was operating at 80 oC. and I
had to leave the cell running overnight, any thermal creep could have resulted in the cell boiling, which would have been a bit of a disaster to say
the least. I was forced to revert to my constant current supply which has a maximum output of 3.5 Amps. I used this for the rest of the run, which
lasted for 33 hours, nearly 3 times the length of the last run. The cell was stirred at about 550 rpm., about 5 times faster than the previous
attempt.
At the end of the run, 25g of solid KCl was added and heating and stirring continued for a further 15 mins. The cell was then slowly cooled to room
temperature and then placed in a refrigerator at about -5 oC. After several hours the cell was tentatively opened, and the dark supernatant liquid was
poured off. Lining the bottom of the container to a depth of about 15 mm. was a mass of aciculate K-permanganate crystals along with some remnant
brown MnO2.H2O "crud". I tried to get some pictures, but the whole mass was slowly collapsing into the murk every time the container was moved. The
flash photo reflected off the insides and a shot from further out wouldn't focus properly. Any way, I scraped it all out and left it in a fine
kitchen sieve, in the fridge, to drain overnight.
Next morning, I washed most of the "crud" off the crystals, rather stupidly using room temperature water instead of ice cold water. This resulted in
some of the finer grained "product" being washed through. The crystals were then put in a desiccator under vacuum for a few hours. With the sun coming
out, I then air dried them for a few hours, they are now back in the desiccator. I haven't weighed them yet as they are still damp, it won't be a
great yield, but at least I'm making some progress.
Maybe it just requires more current for a longer time.
I still have the option of concentrating the remaining solutions and trying to extract a bit more K-permanganate, I'm still thinking about the best
way of doing this.
Image 1: Cell with new lid, operating at nearly 80 oC.
Image 2: K-permanganate crystals, still slightly moist, after air drying. Watchglass is about 100mm. diameter.
EDIT: Well, the K-permanganate has dried overnight in a desiccator, the crystals have a slight "dusting" of brown MnO2. The weight was 6g woohoo! The
theoretical amount of K-permanganate from this run should have been over 30g. In retrospect, if I had known there was a cluster of crystals in the
bottom of the cell, I would have left it in the refrigerator for a much longer time. The dry air would have promoted evaporation and there would have
been a bigger yield. I have added a few seed crystals to my remaining solutions to see if I can extract some more K-permanganate!
Regards, Xenoid
[Edited on 18-9-2007 by Xenoid]

|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
As usual, congratulations, Xenoid. You got the spiky crystals. The Japanese gentlemen have been vindicated.
Sure, the yield seems poor. But the principle is proven. Same as with my earlier wet methods up top of this thread. I intend to do a carefully
measured run again on that, using what I consider the best methodology in light of my experience to date. I hope you will do the same with your
fusion/electrolysis method.
As my old drill sergeant used to say, "The easy way ain't easy, but the 'ard way's bloody 'ard" Producing prmanganate is like that.
Regards, Der Alte .
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Refinement and Optimisation
@DerAlte and Xenoid
Lovely work.
I look forward to seeing the refinements we all hope you will be making.
The electrolytic process looks the most promising for small scale prodution. Perhaps the secret may be to find a way to precipitate the KMnO4 without
contaminating the solution with chloride or whatever so the solution can be reprocessed.
Would it be better to do the precipitaion with KOH? (Assuming it is available). I have a little solid, but have not seen any easily available sources
unless one counts chemical suppliers and their high altitude pricing. Did someone here mention an OTC source of 40% solution?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
K2CO3 is another alternative to KCl. It's sold in pottery supply stores as potassium carbonate or pearl ash.
Remove some of the solution from the cell, add a hot saturated solution of K2CO3 in excess, which will drop out any Mn(II) present. Filter while hot,
return solids to the cell, cool filtrate and filter off KMnO4, return filtrate to the cell.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by not_important
K2CO3 is another alternative to KCl. It's sold in pottery supply stores as potassium carbonate or pearl ash.
|
If only this was the case. I don't think any pottery suppliers in NZ carry K2CO3. I've tried to find it in the past. It's actually only rarely used by
potters, I think it causes cracking of the glaze. They tend to use K-feldspar (orthoclase) in their formulations!
Regards, Xenoid
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Optimisation
Do you think the KMnO4 produced was hydrolysing while the cell was cooling? If so, and if it is an equilibrium mix, then I expect so, then rapid
cooling after K addition would help.
Further on the equilibrium issue, if the hydrolysis is proceeding in the solution , then the oxidation needs to exceed the hydrolysis rate by as
large a margin as possible. Could the current per volume be a factor? ie, the oxidation is not proceeding fast enough to stay ahead?
Of course, the higher current may give heating issues and require active cooling.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ ciscosdad
Hi, I'm not convinced that K-permanganate hydrolises all that quickley in alkaline solutions. I would prefer slow cooling to try and get reasonable
sized crystals which make separation quite simple (with a fine sieve). I think the electrolysis should be done at a higher current. The Japanese
boffins used 20 Amps in a 1 litre container with an anode current density of 40 mA / cm^2. In contrast my current density was only about 12 mA / cm^2,
(3.5A and anode area of about 300 cm^2). I wasn't too worried about this, because the commercial cells operate as low as 5 or 10 ma / cm^2. But since
this procedure is a little different, higher current densities may be called for. That is why I tried to run the last cell at 8 Amps. Unfortunately,
one really needs a constant current supply for this type of electrolysis, otherwise it is impossible to keep the cell at a stable temperature. I'm
currently (no pun intended) looking out for a suitable supply!
Regards, Xenoid
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Current Control
This problem of Xenoids applies to chlorate making as well.
The computer power supplies we all have are great for supplying nice fixed 5volts, but there is a problem controlling the current. A variable
resistor in series comes to mind, but the resistance range we want is a few tenths of an ohm.
It occurred to me that a water resistor may do the trick to lose that critical 2 volts or so.
Has anyone seen anything that will accept a couple of hundred watts if required at around 20 - 30A?
Google gives lots of hits, but all High voltage or high power (motor start etc).
How stable can these devices be? They will not be much use if they vary too much over time (heat or electrode erosion).
Stainless Steel electrodes and Sodium Carbonate or Hydroxide would be better than salt I would think, with a little Dichromate as a depolariser.
I have visions of a 20 litre drum (or 200l  ) full of water with a kilo or so of
Na2CO3 and 2 long SS electrodes hung off the sides. Vary the position and/or the electrolyte concentration to change resistance. ) full of water with a kilo or so of
Na2CO3 and 2 long SS electrodes hung off the sides. Vary the position and/or the electrolyte concentration to change resistance.
Do any of our electronics guys have any input?
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Actually, constant current isn't really required for chlorate making because the temperatures are relatively low. Sure the chlorate cell may get hot,
but the temperature rises until the cell is at equilibrium with the surroundings. Some people put their cells in water, but personally i've never had
a problem, running a 5 litre cell at about 15 - 20 amps from a 5 volt computer supply, the cell tops out at about 50 - 60 oC.
The problem with a permanganate cell is that you have to apply external heat as well as the heat from the electrolysis. With the hotplate cycling, any
increase in external heat causes a decrease in resistance of the cell, so it draws more current, causing more heating, etc. etc. It is like trying to
balance something on a knife edge. Slight increase in heat and the cell runs away and starts boiling, too little heat and the cell drops below optimum
permanganate production.
I'm actually kicking myself, because a few months ago there were a couple of 100 Amp constant current power supplies for sale on the local online
trading site. I decided not to bid because at that time I couldn't really see a use for them. Now I'm just hoping something similar will come up
soon!
I think I've mentioned it before, in another thread, but if you do need a resistor for your chlorate cell, a stripped gouging rod has a resistance of
about 0.2 ohms and makes a useful high wattage variable resistance for limiting current!
Regards, Xenoid
[Edited on 20-9-2007 by Xenoid]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
OMG... a cell... in series with a water resistor...
It's like...well fuck...why don't you put another chlorate cell in series with it then!?...
I use a chunk of steel coat hanger. Brass braze copper wires onto the end. Solder won't do, for what should be obvious reasons. Of course I don't
have as nearly stable a power supply (OC = 8V or so, and the characteristic probably isn't perfectly resistive due to the choke input filter), but the
ballasting effect is notable.
A stick or TIG welder, set to DC output and relatively low current (many go down to "only" 40A), would suffice.
Tim
|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Point taken Tim.
However, the voltage across any extra cell(s) is fixed, and is probably inconveniently just a bit much to fit into the available 5v. I have played
with all sorts of combinations of series and parallell arrangements, without success, unless I have a variac in there. Perhaps I'm just too fussy.
The variable resistor suits my frugal approach to this. The use of a gouging carbon @~.2ohm (Thanks Xenoid!) is perfect.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ ciscosdad
Here are a couple of my 0.2 ohm resistors, not sure what wattage they would handle, at least a 100 W I guess. I mounted the rods in tool clips (Terry
Clips) and made some connectors from strips of SS squeezed around 9.0 and 9.5 mm. drills using a vice and hammer. Make sure the connectors grip the
rods tightly when the screws are done up. Actually, in retrospect, 2 or 3 mounted side by side on a single board would be a better idea than the
individual mounting.
Regards, Xenoid

|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Well, I'm a sucker for punishment.....
.... and against my better judgement I decided to have another go at K-permanganate.
I've brewed up another batch of (hypo)manganate, I have however, made a couple of changes to the procedure used in the first fusion.
Firstly, I dispensed with the water, (NaOH as 50% solution). The only reasons I could see for doing this were that it produced better mixing or
demonstrated that recycled NaOH solution could be used in the procedure.
Secondly, I replaced NaNO3 with KNO3 this should provide sufficient K+ ions in the system to alleviate the need to perform the double dissolution at
the end of the electrolysis.
The procedure is the same as given earlier in the thread, and proceeded smoothly, without any fumes or smell. The final yield was about 250g from .5
moles MnO2 (pottery grade), 3 moles NaOH and 1 mole KNO3. This is enough for 2 runs in my 500 ml. cell.
Image 1: Melt proceeding smoothly.
Image 2: Product after cooling.
Regards, Xenoid

|
|
|
ciscosdad
Hazard to Self
 
Posts: 76
Registered: 6-2-2007
Member Is Offline
Mood: Curious
|
|
Thanks Xenoid.
I like the look of your resistor design. Simple and effective.
We are all looking forward to the results from your new batch. Go to it.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Last, final, ultimate effort!
I've finally run a batch of the last manganate fusion through my cell! This is my final effort!
The results were mildly encouraging, but it would seem to me that one would have to be pretty desperate to make your own permanganate. If you can
aquire the MnO2, the sodium or potassium hydroxide, and the sodium or potassium nitrate required for this process, then you can probably aquire the
potassium permanganate as well!
Half the quantity of manganate made in the last fusion ~125 g. was put in my 500 ml. cell (see above). Stirring was set for 500 rpm and the
temperature ranged from about 70 - 80 oC.
I put together a simple transformer based power supply, running of a variac and set the current for 10 Amps. This current is nearly 3 times what was
used in the previous run and upped the current density from ~ 11 mA/cm^2 to ~ 30 mA/cm^2. The cell was run for about 24 hours.
The cell was slowly cooled overnight and then placed in a refrigerator for 2 days at about -5 oC. When the supernatant liquid was poured off there
was a mass of fine crystals on the bottom of the cell! The crystals were treated as described in the last procedure (see above) however this time I
washed them with ice-cold water. During the drying process some of the crytals seemed to partially redissolve and formed fine crusty material on
further drying. This remained K-permanganate however, as can be seen when a few tiny grains were dropped in water to test it.
The watchglass is 150 mm. in diameter. Final dried weight was about 17 g. almost 3 times the previous effort!
So, it would seem that more electricity is better for a simple cell of this type!
Regards, Xenoid

|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
nice but chlorate is still more fun to make. I turn edible salt into a poisonous powerful oxidizer . Last time I looked the Menard's by my house still has 5 pounds of KMnO4 for $17. . Last time I looked the Menard's by my house still has 5 pounds of KMnO4 for $17.
Fellow molecular manipulator
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by chloric1
nice but chlorate is still more fun to make. I turn edible salt into a poisonous powerful oxidizer . Last time I looked the Menard's by my house still has 5 pounds of KMnO4 for $17. . Last time I looked the Menard's by my house still has 5 pounds of KMnO4 for $17. |
Duh.....! I too make chlorate (and perchlorate) and can buy permanganate for a similar price.... so what! The point of the thread was to try and find
a practical way for the amateur chemist to make it at home. I'm not entirely sure this procedure is all that practical, though!... 
Regards, Xenoid
|
|
|
| Pages:
1
..
6
7
8
9
10
..
17 |