| Pages:
1
2 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I have a bunch of ~70% ammonium nitrate ~30% ammonium sulfate fertilizer that I am hoping to separate into relatively pure NH4NO3 and (NH4)2SO4. I'm
wondering if it would be possible to use a system similar to the one not_important uses in the above post?
I was also wondering is I could add something else to the water to increase the solubility difference between the two salts. I'm thinking an alcohol
or polyol might be helpful.. According to this ammonium sulfate's solubility is substantially reduced by the presence of various polyols. No actual numbers though. So this brings me to the
question, does anyone have some actual data on water, alcohols or polyols and NH4NO3 and/or (NH4)2SO4 systems?
Basically I'm trying to find the optimum mixture that gives the largest solubility difference while still being cost effective and practical. Neat
methanol seems like a possibility but has disadvantages like flamability (especially when working with large amounts  ), cost/availability, and NH4NO3 not being very soluble in it. So I was thinking a
MeOH+H2O mix might be more practical, but I have no data on (NH4)2SO4 or NH4NO3 solubility in those mixtures, so I have no way of knowing what would
be a suitable mixture.. ), cost/availability, and NH4NO3 not being very soluble in it. So I was thinking a
MeOH+H2O mix might be more practical, but I have no data on (NH4)2SO4 or NH4NO3 solubility in those mixtures, so I have no way of knowing what would
be a suitable mixture..
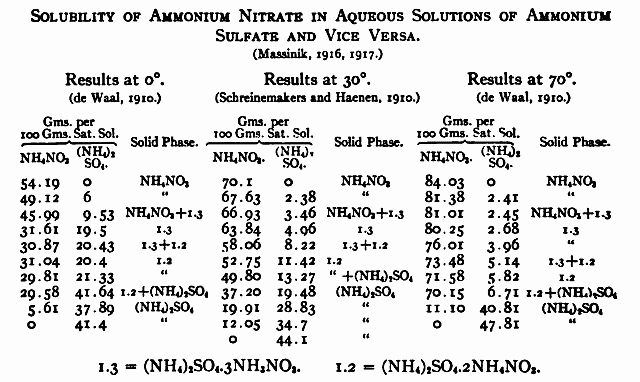
EDIT: I have finally been rewarded for wallowing in patents for hours.. I have attached several gems of solubility data.
Ethylene glycol is looking like a very good canidate. It's cheap and extremely accessible, and is not particularly flammable or volatile. I don't know
for sure, but I would guess that (NH4)2SO4 has a similar solubility in EG compared to MeOH. Maybe 1g/100ml EG at most? Where NH4NO3 is going to have a
solubility of over 50g/100ml EG when warm. That looks like it could very usable. The addition of some water would even further increase the NH4NO3
solubility, but I wonder if the solubility difference between it and (NH4)2SO4 would be better?
[Edited on 3-1-2009 by 497]
Attachment: solubility data.doc (134kB)
This file has been downloaded 552 times
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Potential problem with water-alcohol (or polyol) mixes is a phase separation into water+salt rich and alcohol-rich layers.
It is possible that the solubility of ammonium sulfate in EG would be higher than in MeOH, should be easy to test.
Another difficulty with EG is what you list as a advantage - its low volatility. Once you've got NH4NO3 crystallising out of it, how do you dry them?
The EG will hang around for some time, necessitating recrystallising from water at least once.
Is that pure EG, or an antifreeze which often has additives?
I would start with making a solution of the fertilizer, then adding enough CaCO3 or Ca(OH)2 to react with the sulfate plus a slight excess. Boil
gently to drive off the released ammonia, adding water to keep the volume constant, until little ammonia is given off. Either move to a water bath or
remove from heat and wrap the container in insulation to slow its cooling; let settle for awhile, then decant through a filter. Treat the cold
filtrate with ammonium carbonate or NH3+CO2, heat to around 40 C, cool, filter. Bring filtrate to a boil to drive off excess of NH3+CO2, cool,
decant-filter.
[Edited on 4-1-2009 by not_important]
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
| Quote: | Originally posted by not_important
Len, I did something dimly similar. I placed a large jar inside a fish tank, put a large test tube in the center of the jar and clamped it there,
filled the jar with fertiliser grade ammonium sulfate, filled the test tube with mineral oil and dropped in a cartridge header, and poured a cm or so
of fine sand on top of the sulfate. I then filled the tank with DW. The water was higher that the top of the jar, but the test tube stuck out of the
water. Finally I dropped in a bubble stone hooked up to an aquarium air pump, and added aqueous ammonia until pH 8.
I then applied power to the cartridge heater, slowly, until the oil reach 80 C. I then covered the tank and left it.
The warmed water inside the jar dissolved some of the sulphate. Eventual diffusion lead to the tank being filled with a saturated solution of the
sulfate, and then to crystals being deposited on the tank walls, cooler than the heater containing jar.
Eventually most of the sulfate in the jar dissolved. I turned off the heater, lifted the jar out of the tank and syringed out most of the solution in
the jar. The added fresh crude sulfate to the jar, and capped with fine sand again. Back in the tank, add aqueous ammonia again, turn the heater back
on.
I ran three 20 Kg sacks of ammonium sulfate through, got about 52 kg of recrystallised white (NH4)2SO4 off the tank walls and liters of the saturated
solution that I used in the garden for the next several years.
The cold/hot solubility ratio of ammonium sulfate was low enough that I'd gotten frustrated with typical recrystallisation. This way I lost less to
the mother liquor, and didn't have to put uf with filtering the solution.
[Edited on 13-8-2007 by not_important] |
That sounds like a good system, especially if you can find the fishtank used at a garage sale or on craigs list. I only have 2 questions. What is a
cartridge heater? And what volume of jar did you use to what volume of fish tank?
I have been wanting to further explore the diffusion techniqu for some time. Just have not had supplies or time to plan the setup since leaving it
undisturbed at constant temperature for days or weeks is a little challenging but certainly possible.
My diffusion experiments would consist of precipitation by diffusion to form larger crystals. Like crystaline calcium hydroxide or barium
thiosulfate. Just put small lipless cups of your saturated reagent solution with excess solid in the bottom and put both containers in a low form
large container and carefully add water until the level covers both containers with several centimeters of water and pour melted paraffin over the
water surface to block out dust and breezes and set aside for a few weeks. Ions should diffuse out of the beakers and deposit crystals of the least
soluble component when solubility product is exceeded.
Fellow molecular manipulator
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
Once you've got NH4NO3 crystallising out of it, how do you dry them? The EG will hang around for some time, necessitating recrystallising from water
at least once. |
Couldn't you just wash it with something like petroleum ether or acetone to get the EG off?
| Quote: |
Is that pure EG, or an antifreeze which often has additives? |
Some antifreezes are virtually all EG, a little bit of diethylene glycol, probably a small bit of water, and sometimes a corrossion inhibitor like
sodium phosphate in small amounts, and of course the bright green dye (fluorescin?). Nothing that should interfere.
| Quote: |
I would start with making a solution of the fertilizer, then adding enough CaCO3 or Ca(OH)2 to react with the sulfate plus a slight excess. Boil
gently to drive off the released ammonia, adding water to keep the volume constant, until little ammonia is given off. |
I was really hoping to avoid dealing with CaSO4.... But if all else fails, I will go that route. Actually I have some Ca(NO3)2 fertilizer that will
yield even more NH4NO3 when reacted with the sulfate... Maybe I'm overestimating the trouble this would be.. all I know is last time I had to deal
with CaSO4 I vowed never to do it again.
EDIT: I tried it on a small scale today. I mixed 192g Antifreeze (straight out of the bottle) with 139g Fertilizer (straight out of the bag). I heated
it in a boiling water bath for at least 30 minutes while stirring. It looked like a large portion of the solids dissolved. The surface of the glycol
was covered with an ugly brown scum. Then I filtered it through a paper towel, which caught the scum nicely and left me a nice only slightly foggy
solution. It worked pretty well, the filtering went quickly. The glycol solution gained about 57g (plus some that was lost as residue, etc). I took a
small aliquot of the filtrate and left the rest in the water bath to slowly cool and hopefully give some decent crystals.
I set the aliquot (~2ml) in the freezer for a few minutes. It solidified with precipitated tiny needle crystals. I then let it warm back up to room
temperature, where most of the precipitate redissolved. I then tried mixing in some toluene. As I not_important said, the two did not mix at all. So I
poured off most of the toluene and let the rest evaporate. Then I tried MEK. Same thing happened. So I evaporated off the MEK and tried denatured
ethanol (~10ml). This worked nicely, a substantial amount of fine white precipitate came out. Since AN is substantially soluble in ethanol using
isopropanol is another possibility that would decrease the solubility even more. I'll have try it and see if they mix. If not, then a mixture of
isopropanol and ethanol would probably be the ideal choice.
[Edited on 4-1-2009 by 497]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | What is a cartridge heater? And what volume of jar did you use to what volume of fish tank? |
http://www.watlow.com/products/heaters/ht_cart.cfm
and roughly 1/2 to 1/3 the total volume of the tank. Smaller jar means more of the first load ends up in the solution, which is why you reuse it
several times.
| Quote: | | Couldn't you just wash it with something like petroleum ether or acetone to get the EG off? |
Not non-polar solvents like pet ether, basically if the solvent isn't moderately soluble in water then EG is likely only very slightly to insoluble in
the solvent at room temperature or close to that. Acetone might work, but AN is slightly soluble in that so a tiny bit will be lost.
| Quote: | | I was really hoping to avoid dealing with CaSO4.... But if all else fails, I will go that route. Actually I have some Ca(NO3)2 fertilizer that will
yield even more NH4NO3 when reacted with the sulfate... Maybe I'm overestimating the trouble this would be.. all I know is last time I had to deal
with CaSO4 I vowed never to do it again. |
I'd stick with the mixed ammonium nitrate/sulfate, as it has no other uses while Ca(NO3) can be used to make nitrates of metals with soluble sulfates;
magnesium nitrate is useful for cleaning carbon and organic crud out of glassware and can be had by mixing Epsom salts and calcium nitrate.
A bit of boiling often coarsens the calcium sulfate. After that letting it settle for some time with occasional light tapping of the container,
followed by decanting or siphoning of the supernatant liquid away from the precipitate, usually works well. Afterwards filter the liquid to remove
small amounts of suspended sulfate, don't try to filter the bulk of the precipitate with conventional means.
To get the remaining liquor from the calcium sulfate, I dumped the gloppy precipitate into a tofu or cheese press lined with an outer layer cheese
cloth and an inner (touching the precipitate) layer of muslin, calico, or cambric of plain cotton or linen. Have it set up over a plastic basin,
scoop in the precipitate, drop on the top and weights, and go away for some hours or longer.
You can be frugal and wash the sulfate precipitate well, spread it in thin layers and allow it to dry, break it up into coarse chunks, and dry at
190-230 C to get a decent drying agent. If you keep at at 30C or cooler it's a good as H2SO4 for drying most gases, although it will absorb ammonia
and small amines to some extent.
BTW - nice research on your approach. That data is around but buried in difficult to find places, so DIY is often the way to go.
[Edited on 5-1-2009 by not_important]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: |
Not non-polar solvents like pet ether, basically if the solvent isn't moderately soluble in water then EG is likely only very slightly to insoluble in
the solvent at room temperature or close to that. Acetone might work, but AN is slightly soluble in that so a tiny bit will be lost.
|
Yes I found that experimentally.. But I think washing with EtOH/iPrOH will do the trick.
The beaker of filtrate (from my above edit) has been sitting near room temperature for about 20 hours now. It has grown some amazing crystals on the
bottom and lower sides. They consist of clear needles that are up to 1.5-2 cm long, and maybe 1 mm thick. Very pretty. Now I have it in the
refrigerator (and later freezer) to see how much bigger they'll grow. I have to say I'm surprised, I expected to get a big gelatinous mass of fine
crystals.
EDIT:
After sitting in the fridge for a few hours and then the freezer for a few more hours, this is what I ended up with:



The green tinge is from the dye in the EG residue.
[Edited on 5-1-2009 by 497]
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
not-important That is awesome! When I get back to the USA, I will call a watlow distributor. Nice touch with the mineral oil as aa heat
dispersant. I might want to get some silicone oil from chemistrystore.com as I think it can work at higher temperatures if needed.
Just should mention that ammonium nitrate is appreciably soluble in 40% aqueous alcohol. Potassium sulfate is only 0.5% soluble in the same. That
might be easier and cheaper than using straight methanol but I would like to try both.
Fellow molecular manipulator
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Woops, here's the picture I meant to post instead of the second one. Gives a little better view. Of course the edit function has expired...

|
|
|
KoiosPhoebus
Hazard to Self
 
Posts: 51
Registered: 23-1-2023
Member Is Offline
|
|
Quote: Originally posted by DeAdFX  | | Quote: | Originally posted by not_important
The problem with the calcium nitrate/ammonium sulfate route, having done this when I was 12, is that calcium sulfate has its solubility increased by
ammonium salts. If you take ammonium nitrate made this way, and recrystallised several times, it still leaves a fair residue of calcium oxide behind
when you heat a bit to decomposition. Depends on your application if the calcium is a problem or not. |
Does Calcium Phosphate suffer the same downfalls? Triammonium Phosphate should be a fairly easy to find at a large farming supply store correct?
I also wonder if its possible to make ammonium nitrate/nitric acid using mono or dihydrogen phosphates as can be done with bisulfate. This is just in
case there is no Triammonium phosphate.... I know that the hydrogens on the phosphate are of going to be of varying strength but am uncertain of how
it would apply to the problem...
Bisulfate is a weaker acid than nitric acid when I look at the acid strength charts. I guess the reaction proceeds because a precipitate is formed?
[Edited on 13-7-2007 by DeAdFX] |
Bit of an old thread, but I came across this while searching to see if anyone
had written up my method for preparing ammonium nitrate from diammonium phosphate (https://www.sciencemadness.org/whisper/viewthread.php?tid=15...) and I thought I'd reply.
Yes, you can use the diammonium phosphate as dicalcium phosphate (calcium hydrogen orthophosphate) has very low solubility in water (0.018g/100mL). In
fact it has lower solubility than calcium sulphate (0.26g/100mL).
I would not recommend using the monoammonium phosphate (aka ammonium dihydrogen orthophosphate) as it produces monocalcium phosphate which is somewhat
soluble in water (1.8g/100mL). Some literature (e.g. https://pubs.acs.org/doi/abs/10.1021/acs.iecr.5b02100) even argues that monocalcium phosphate itself is much more soluble than that, it's just
that some of it decomposes into phosphoric acid and dicalcium phosphate when dissolved.
|
|
|
| Pages:
1
2 |
|