Tom Thumbs plum
Harmless

Posts: 5
Registered: 19-8-2018
Member Is Offline
|
|
Hydroxylamine alternatives or synthesis
Hello everyone I've been a long time lurker of SM.
One thing I could not find by UingTFSE is are there any alternatives to hydroxylamine for conversion of aldehydes to oximes?
Nitromethane and sulfuric are hard to obtain in my area. The rc fuels have less and less quantities of NM in them. The last gallon of 25% I had turned
out around 10% although add a few % for azeotrope.
If it was more than this it wouldn't be a problem as the hcl method works and is simple. But for 10%/gallon @ £30/gallon and the time for
distillation it dosent seem worth it.
Any other methods I have seen involve so2 which puts me off these routes.
Are there still sources of hydroxylamine as 50% solution available in uk? I've searched and searched through msds and can't find a source.
Any help is greatly appreciated.
Tom.
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
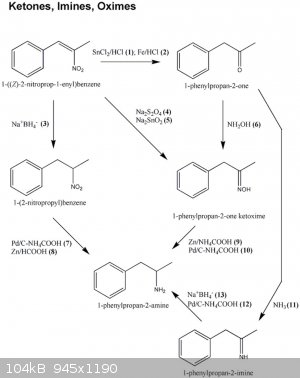
Look at this. Is seems you can reduce nitroalkene with na2s2o4 or (i believe) using Thiourea dioxide to oxime.
[Edited on 19-8-2018 by mackolol]
[Edited on 19-8-2018 by mackolol]
|
|
|
Tom Thumbs plum
Harmless

Posts: 5
Registered: 19-8-2018
Member Is Offline
|
|
Thanks mackolol, I.ll give that a go.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I suspect this path works but as to the quantity that can be reaped, uncertain.
First, generate the hydroxyl radical •OH (alone or together with the hydrogen atom radical •H or even the carbonate radical anion •CO3-) in the
presence of ammonia gas (no oxygen):
•OH + NH3 --> H2O + •NH2
Or:
•H + NH3 --> H2 + •NH2
Or:
•CO3- + NH3 --> HCO3- + •NH2
And finally:
•OH + •NH2 = NH2OH
where the last reaction may be reversible if you using photolysis, for example, to generate the starting radicals. Also expect other products as well,
like:
•NH2 + •NH2 = N2H4
•H + •H = H2
......
Note, the photolysis of a boiled (removing O2) carbonate solution can produce the carbonate radical and more (see this 1969 refernce: https://pubs.acs.org/doi/abs/10.1021/j100909a029?journalCode... _
CO3(2-) + hv→ •CO3- + e-(aq)
H2O = H+ + OH-
e-(aq) + H+ = •H
.....
Also, the UV photolysis of aqueous N2O:
N2O + hv --> N2 + •O- (see https://www.ncbi.nlm.nih.gov/pubmed/30074775 (
•O- + H2O = OH- + •OH
OH- + UV --> •OH + e- (see https://pubs.acs.org/doi/abs/10.1021/j100864a044?journalCode... )
[Edited on 19-8-2018 by AJKOER]
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Hydroxyl radical can be generated by Fenton's reagent. It's just feso4 solution mixed with h2o2. But this process is extremely violent as it creates
free radicals. Fenton's reagent is commonly used to oxidise compounds or adding hydroxyl group. For example oxidation of benzene yields phenol,
oxidation of thf yields gbl, oxidation of benzyl alcohol yields benzaldehyde. So the reaction is simply oxidation of ammonia with Fenton's reagent i
guess AJKOER. So if it works as that you can skip process of oxidation of nh3 which would be oppresive as the ammonia is gas and the process generates
a lot of heat and just oxidise the ready amine (or rather imine created by reaction of ammonia with ketone). Maybe at this point (of having primary
imine formed by reacting ammonia with ketone or aldehyde) you can try oxidising it with other oxidisers such as kmno4 (but it can over oxidise the
imines for example to nitro compounds). Unfortunately i don't have any papers describing oxidation or even properties of primary imines. If someone
has i would be very thankful.
[Edited on 19-8-2018 by mackolol]
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
This site presents a lot of ways to oxidise primary amine to oxime for example with meta- Chloroperoxybenzoic acid.
https://www.organic-chemistry.org/synthesis/C2N/oximes.shtm
[Edited on 19-8-2018 by mackolol]
|
|
|
MichaelStars
Harmless

Posts: 1
Registered: 19-8-2018
Member Is Offline
|
|
Ajkoer thank you
|
|
|
Tom Thumbs plum
Harmless

Posts: 5
Registered: 19-8-2018
Member Is Offline
|
|
Many thanks 
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Hydroxylamine HCl: http://www.orgsyn.org/Content/pdfs/procedures/CV1P0318.pdf
I prefer the prep of hydroxylamine sulfonate solution from http://www.orgsyn.org/Content/pdfs/procedures/CV2P0204.pdf which can be easily modified to use metabisulfite.
At the expense of yield, I have simplified the purification by using an excess of MEK instead of acetone. The MEK needs to be stirred to get it into
the hydroxylamine solution. The oxime is a liquid and has lower solubility compared to acetone oxime. I may have added salt to saturate the solution
after stirring overnight as well, but I'm not sure where my notes are. I seperated the oxime mechanically and slowly distilled MEK-water azeotrope
from the mixture with an excess of HCl. This distillation needs to be very slow and possibly through a short column. Compared to hydrazine prep where
distilling MEK ketazine and sulfuric acid can be done as fast as possible, the oxime is much more hydrolysis resistant.
[Edited on 19-8-2018 by UC235]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mackolol  | Hydroxyl radical can be generated by Fenton's reagent. It's just feso4 solution mixed with h2o2. But this process is extremely violent as it creates
free radicals. Fenton's reagent is commonly used to oxidise compounds or adding hydroxyl group. For example oxidation of .....
[Edited on 19-8-2018 by mackolol] |
The introduction of an oxygen source (like H2O2) is likely undesirable for my proposed reaction system (more side reactions). However, one could
employ aqueous NH2Cl (albeit even more problematic than ammonia water) in the redox:
Fe(ll) --> Fe(lll) + e-(aq)
NH2Cl (aq) + e-(aq) --> •NH2 (aq) + Cl- (aq)
Net reaction: Fe(ll) + NH2Cl (aq) --> Fe(lll) + Cl- + •NH2 (see, for example, https://www.ncbi.nlm.nih.gov/pubmed/11878380 and also https://www.ncbi.nlm.nih.gov/pubmed/11871569 )
which apparently forms the •NH2 radical without consuming hydroxyl radicals (as occurs in the reaction of ammonia with •OH).
Next, to get the required hydroxyl radical, one could try to use a fenton-type reaction between Fe(ll) with added HOCl assuming pH is below 7:
Fe(ll) + HOCl --> Fe(lll) + •OH + Cl- (see, for example, "Fenton chemistry in biology and medicine*" by Josef Prousek, and related reaction (15)
on page 2330)
Then, to some extent, the possible reaction:
•NH2 + •OH = NH2OH
However, a problem with this particular fenton-type system approach, in my assessment, is that in the classic fenton reaction with added NH2OH, it
favorably recycles the Fe(lll) to Fe(ll) (see, for example, https://www.sciencedirect.com/science/article/pii/S138358661... ), the issue being that recycling iron ions, while good for a fenton system, does
apparently result in the consumption of the NH2OH itself!
2 NH2OH + 4 Fe(lll) --> 4 Fe(ll) + N2O + H2O + 4 H+
So a fenton path to source hydroxyl radicals for hydroxylamine creation is likely not advisable (hence my alternate recommendations).
[Edited on 19-8-2018 by AJKOER]
|
|
|
Tom Thumbs plum
Harmless

Posts: 5
Registered: 19-8-2018
Member Is Offline
|
|
UC235 i have pondered over that first link a few times but the use of so2 puts me off.
I have wondered would an excess off bisulfite work instead of bubbling so2?
Second link looks interesting.
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
But AJKOER does it necessarily need two radicals nh2 and oh? Isn't hydroxyl radical reactive enough to oxidise nh3 to nh2oh?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mackolol  | | But AJKOER does it necessarily need two radicals nh2 and oh? Isn't hydroxyl radical reactive enough to oxidise nh3 to nh2oh? |
Yes, the following two reactions are sufficient but not the whole picture depending on how the hydroxyl radicals are generated and possible other side
reactions:
•OH + NH3 --> H2O + •NH2
•NH2 + •OH = NH2OH
And, as I noted, even the last reaction above may be subject to reversal under photolysis that was employed to produce hydroxyl radicals.
Further, I also detailed above how a fenton derived hydroxyl radicals are likely not a recommended path either due to the ability of NH2OH to reduce
Fe(lll) to Fe(ll) in a fenton system per the self destructive reaction:
2 NH2OH + 4 Fe(lll) --> 4 Fe(ll) + N2O + H2O + 4 H+
Bottom line, I have not come up with a possible path to implement the above two key reactions that I would recommend as worthy of investigating so
far.
[Edited on 19-8-2018 by AJKOER]
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
But do oximes also get reduced by Fe(III)? What if we oxidise primary amine -NH2 or imine =NH with Fenton?
|
|
|
Tom Thumbs plum
Harmless

Posts: 5
Registered: 19-8-2018
Member Is Offline
|
|
I just found this, a few methods I have never seen mentioned anywhere else.
http://encyclopedia.jrank.org/HOR_I25/HYDROXYLAMINE_NH2OH.ht...
" E. Divers obtains it by mixing cold saturated solutions containing one molecular proportion of sodium nitrate, and two molecular proportions of acid
sodium sulphite, and then adding a saturated solution of potassium chloride to the mixture. After standing for twenty-four hours, hydroxylamine
potassium disulphonate crystallizes out. This is boiled for some hours with water and the solution cooled, when potassium sulphate separates first,
and then hydroxylamine sulphate. "
Another method is electrolysis with sulfuric, nitric and lead.
Edit... here is another method by Divers similar to the one above that looks very promising and simple.
https://archive.org/stream/catalyticprepara00jone/catalyticp...
" Divers and Haga'have succeeded in obtaining Hydroxylamine
in the following manner. If sodium sulphide and sodium nitrite
in solution are mixed, then acidified and boiled, Hydroxylamine is
formed, and when the sulphide is in the proportion of 2 mols to
1 mol of the nitrite, and the hydrochloric acid added very slowly,
almost all of the nitrogen is found on titration with iodine to
have been converted into Hydroxylamine.
NaN02 -r 2Na2S03 + HOH t RHCL — ^NH20H,HC1 3Nacl 2NaHS04
On evaporating the acid solution, separating the sodium salts by
absolute alcohol and evaporating again, Hydroxylamine sulphate
separates out. Further experiment proved that sodium meta-
sulphide used with sodium nitrite gave the best yield of Hydroxy-
lamine sulphate. "
With what you all have given me and the routes that I've found I have a lot to work on.
Thanks,
Tom.
[Edited on 20-8-2018 by Tom Thumbs plum]
|
|
|