| Pages:
1
..
31
32
33
34
35
..
43 |
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
byko3y: Noted. Although I continue to insist that the differences in reactivity between SCl2 and S2Cl2 can be considered analogous to C2H4Cl2 vs
CH2Cl2. SCl2 has a much smaller dipole moment because the two S-Cl bonds are in opposing directions. Likewise SOCl2 and COCl2 have large dipole
moments because the C=O and S=O bonds are highly polarized.
It seems like the intent was to make anhydrous zirconium (IV) sulfate, Zr(SO4)2, which is both a dehydrating agent and a Lewis acid. Zirconium (IV)
and titanium (IV) both like to form covalent-ish bonds with chlorine, nitrogen and oxygen, and TiCl4 is volatile. However, note that while it is a
metal salt, it's not exactly easy:
". Excess water was evaporated on a water-bath and the resulting sample was oven-dried at 120 °C for 12 h and calcined at 500 °C for 4 h in air
atmosphere and stored in vacuum desiccator."
I doubt that anhydrous zirconium sulfate can be made by an "easy" method. It's likely that simply adding zirconium to anhydrous sulfuric acid will
result in passivation. In this case the half-reaction comprises 3 moles of Zr(SO4)2 reacting with 5 moles (acid / 2) of water.
http://www.sciencedirect.com/science/article/pii/00406031938...
Notably, the method as reported is consistent with this analysis of the thermodynamics of zirconium sulfate, since Zr(SO4)2 begins to decompose just
above 500 C at 540 C. The half-reactions are Zr(SO4)2 + H2O >> Zr(SO4)2*H2O, Zr(SO4)2*H2O + H2O >> Zr(SO4)2*2H2O, and the one where I know
the energetics, 2 AcOH + ~90 kJ/mol >> Ac2O + H2O. So the heat of hydration of Zr(SO4)2 would have to be at least 90 kJ/[mol H2O], probably
higher as entropy decreases.
[Edited on 15-5-2016 by clearly_not_atara]
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
What about decomposition of an acetate using dry chlorine, related to the production of nitrogen pentoxide:
2AgNO3+Cl2->2AgCl+ N2O5+O2
Enthalpy of formation:
Acetic Anhydride:-625 kJ/mol
Sodium Chloride:-411 kJ/mol
Sodium Acetate:-709 kJ/mol
Assuming we use sodium acetate, the reaction is not favored, but there may be a way to drive the reaction in favor, perhaps by using a silver salt and
then recovering the silver.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Chlorine will probably attack acetic anhydride, since the alpha-protons on anhydrides act similar to ketones, i.e. enolizability and stuff. Silver
acetate can already be used to prepare acetic anhydride by simple heating as long as it's done under the right conditions. Apparently the
AgOAc and surrounding atmosphere has to be completely dry to produce Ac2O because otherwise it undergoes water-catalyzed decomposition to CO2 and
acetone.
There's also a method with zinc acetate that sadly has 25% theoretical yield and a possibility of using copper acetate as well although I've never
seen literature on Cu(OAc)2 decomposition specifically. I'm guessing all of the dry distillation methods require exquisitely anhydrous conditions.
Another possibility if it works might be to react methylene halides with silver acetate to get the acylal (AcO)2CH2 which decomposes to Ac2O in the
presence of catalytic acid. According to this page the bp of methylene diacetate is 164 C:
http://www.apolloscientific.co.uk/display_item.php?id=41521
CH2Cl2 seems much nicer to work with than Cl2, assuming it's actually reactive enough (or you'd have to use the iodide or bromide instead).
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
All possible way for synthesis of methylene diacetate by reaxys
(item 4/36 and 19/36 and is interesting):
| Quote: |
Methylene Diacetate Stock Solution:
Bu4NOAc (11.4 g, 37.8 mmol) and anhyd benzene (60 mL) wereplaced in a round-bottomed flask equipped with a Dean–Stark apparatus. This
solution was refluxed under an argon atmosphere with continuous removal of the azeotrope over a period of 1 h. Then the flask was connected to a
vacuum line to remove the rest of the benzene. Finally, anhyd CH2Cl2 (37 mL) was added to the flask containing Bu4NOAc (11.2 g, 37.1 mmol) under an
argon atmosphere.
This stock solution was allowed to stand at r.t. for 3 d before use and then kept at 4 °C
Boron Trifluoride Mediated Prins Reaction of Methylene Diacetate with Styrenes. One-Pot Synthesis of
3-Chloro-3-arylpropanolsAlper Isleyen, Özdemir Dogan*Department of Chemistry, Middle East Technical University, 06531 Ankara, Turkey
item 19/36
|
Attachment: Methylene Diacetate.pdf (309kB)
This file has been downloaded 939 times
[Edited on 8-2-2017 by Waffles SS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Well now I'm not sure if silver acetate would work or not. Apparently acetate ion will eventually react with CH2Cl2, so the counterion shouldn't
matter *too* much, right?
Tetrabutylammonium acetate would of course be preferable. I wonder if you can get away with tetraethylammonium acetate? But take precautions in
handling tetraethylammonium (full body covering) as its salts are highly neurotoxic and you don't want it on you
|
|
|
BILLBUILDS
Hazard to Self
 
Posts: 82
Registered: 19-3-2016
Member Is Offline
Mood: No Mood
|
|
from wikipedia
| Quote: |
Basic zinc acetate
Heating Zn(CH3CO2)2 in a vacuum results in a loss of acetic anhydride, leaving a residue of basic zinc acetate, with the formula
Zn4O(CH3CO2)6.
|
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by BILLBUILDS  |
from wikipedia
| Quote: |
Basic zinc acetate
Heating Zn(CH3CO2)2 in a vacuum results in a loss of acetic anhydride, leaving a residue of basic zinc acetate, with the formula
Zn4O(CH3CO2)6.
|
|
read page 21
also below article:
Evidence for the formation of anhydrous zinc acetate and acetic anhydride during the thermal degradation of zinc hydroxy acetate,
Zn5(OH)8(CH3CO2)2·4H2O to ZnO
Timothy Biswicka, , William Jonesa, Aleksandra Pacułab, Ewa Serwickab, Jerzy Podobinskib
http://www.sciencedirect.com/science/article/pii/S1293255808...
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Befor I do it, I just wanna ask if anyone tried this:
Succinic acid can be dehydrsted at about 230°C to succinic anhydride, and unlike the acid, the anhydride is liquid 230°C. So my idea is to just mix
the succinic anyhdride in a 1:2 mol ratio with sodium acetat and melt this whole mix. And then you might be able to distill off acetic anhydride. The
succinic acid can then be recycled by dissolving the sodium succinate in the distilling flask and acidify it, to precipitate the succinic acid out.
That can then be dehydrated again by heating it.
As I said, I have no idea if that would work, but I'am planning on trying that out.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
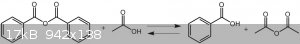
I tried Zinc acetate method and even your idea method by Succinic anhydride,Phthalic anhydride and another anhydride like benzoic anhydride.
According to my experiment reaction of cyclic anhydride with acetic acid will not lead to Ac2O.I dont know what exactly happen but i think
in this case formation of 5 membered ring is main route and Ac2O will not produce.
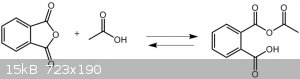
About benzoic anhydride and another linear anhydride we have equilibrium between formation of Ac2O and benzoic anhydride and you can make
Ac2O if you can push reaction to it side.
Attachment: kremann1923.pdf (355kB)
This file has been downloaded 606 times
There are many articles about pyrolysis of Zinc acetate and most of them scale are Micromole.I tried it but i was unsuccessful.You have to heat powder
to about 300C,at this temp huge amount of smoke produce and then yellow liquid start to come that has acetic odor but is not Ac2O.
[Edited on 20-5-2017 by Waffles SS]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Succinic anhydride dehydrates more readily than acetic acid, while acetic acid is more volatile than acetic anhydride. Even benzoic anhydride-acetic
acid pair will give a solution of acetic anhydride in acetic acid.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you want to do that, consider gassing the AcOH/(RO)2O with HCl to distill out AcCl. This can be recombined with sodium acetate which is
particularly preferable if the product is to be stored or it may be used immediately.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by byko3y  | | Succinic anhydride dehydrates more readily than acetic acid, while acetic acid is more volatile than acetic anhydride. Even benzoic anhydride-acetic
acid pair will give a solution of acetic anhydride in acetic acid. |
You are right.This is why acetyl chloride Produce in reaction of benzoyl chloride and acetic acid.Because acetyl chloride is more volatile than
another materials and the equilibrium move to acetyl chloride side.
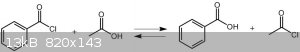
[Edited on 20-5-2017 by Waffles SS]
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by byko3y  | | Succinic anhydride dehydrates more readily than acetic acid, while acetic acid is more volatile than acetic anhydride. Even benzoic anhydride-acetic
acid pair will give a solution of acetic anhydride in acetic acid. |
That might be true, but luckly, succinic anhydride melts at about 230°C, and I imagine that if you put sodium acetat into molten succinic anhydride,
you'll get acetic anhydride distilling out.
Quote: Originally posted by Waffles SS  | I tried Zinc acetate method and even your idea method by Succinic anhydride,Phthalic anhydride and another anhydride like benzoic anhydride.
According to my experiment reaction of cyclic anhydride with acetic acid will not lead to Ac2O.I dont know what exactly happen but i think
in this case formation of 5 membered ring is main route and Ac2O will not produce.
About benzoic anhydride and another linear anhydride we have equilibrium between formation of Ac2O and benzoic anhydride and you can make
Ac2O if you can push reaction to it side.
[Edited on 20-5-2017 by Waffles SS] |
I honestly still don't know why people make acetic anhydride though benzoic anhydride, I mean isn't benzoic anhydride as least as hard to get as
acetic anhydride? And for making only one molecule of acetic anhydride, benzoic anhydride is quite a big molecule, so you also need quite a lof of it
for little acetic anhydride.
Quote: Originally posted by clearly_not_atara  | | If you want to do that, consider gassing the AcOH/(RO)2O with HCl to distill out AcCl. This can be recombined with sodium acetate which is
particularly preferable if the product is to be stored or it may be used immediately. |
You brought me to a quite interesting idea, maybe it's possible to mix acetic acid with succinic anhydride, and then bubble HCl gas into this
solution, which then should react with the succinic anhydride to make succinyl monochlorid, which then makes acetyl chlorid which you can distill out.
And seeing that acetyl chloride is way cooler than acetic anhydride, I'am really eager to try that out.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by theAngryLittleBunny  |
That might be true, but luckly, succinic anhydride melts at about 230°C, and I imagine that if you put sodium acetat into molten succinic anhydride,
you'll get acetic anhydride distilling out.
|
According to what mechanism or reference Sodium acetate will react with molten succinic anhydride and make Ac2O?Did you invent new reaction?
Bigger or smaller molecule is not problem because we dont want to run a plan of Ac2O from benzoic anhydride.We are not looking for economic way for
synthesis AC2O.We are talking about possibility in organic chemistry,Possibility of making Ac2O from Benzoic anhydride or another anhydride.
[Edited on 20-5-2017 by Waffles SS]
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  |
According to what mechanism or reference Sodium acetate will react with molten succinic anhydride and make Ac2O?Did you invent new reaction?
Bigger or smaller molecule is not problem because we dont want to run a plan of Ac2O from benzoic anhydride.We are not looking for economic way for
synthesis AC2O.We are talking about possibility in organic chemistry,Possibility of making Ac2O from Benzoic anhydride or another anhydride.
[Edited on 20-5-2017 by Waffles SS] |
What? I thought the whole point of this thread was to find a way of making acetic anhydride for people who don't havee access to it, people like me
.-.
And I don't have a reference, I don't even know if it would work, it's just an idea of mine I wanna try out, since it would be a very economical way
of making acetic anhydride in theory.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Theory should be true according to chemistry laws and should be logical.This thread based on logical ways for synthesis Ac2O not our wishes.I promise
there are no way for synthesis Ac2O by reaction of Succinic anhydride with sodium acetate because is not logical according to chemistry laws.
This is basic Organic Chemistry
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  | Theory should be true according to chemistry laws and should be logical.This thread based on logical ways for synthesis Ac2O not our wishes.I promise
there are no way for synthesis Ac2O by reaction of Succinic anhydride with sodium acetate because is not logical according to chemistry laws.
This is basic Organic Chemistry |
Seeing that you're 13 years older then me, you probably know that stuff better then I do. I mean seeing that benzoic anhydride could dehydrate acetic
acid, I honestly don't see a reason why succinic anhydride could not react with sodium acetat. I also thought about a methode of recluxing succinic
anhydride dissolved in acetone with calcium acetate, my idea is that it would make calcium succinate and acetic anhydride. But as I said, I still have
to try it. I still would love to know the mechanism in which an carboxylic anhydide dehydrates an carboxylic acid, I'am sure you know it, because I
can't really find anything about it on the internet. I imagine that that is starts with the negative oxygen on the caroboxylate attacking the carbon
in the acid group, since that should be somewhat electrophilic. Because if that's the case, I see no reason why it shouldn't work with an carboxylate
salt.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Previous attempts at making acetic anhydride from copper acetate failed due to the difficulty of preparing anhydrous copper acetate. However it might
be possible to make anhydrous copper acetate by using salt metathesis in a non-aqueous solvent. Anhydrous copper (II) chloride is soluble in ethanol
(~50g / 100 mL), as is potassium acetate (16g / 100 mL according to http://chemister.ru/Database/properties-en.php?dbid=1&id... ) . Potassium chloride is basically insoluble in ethanol. So in order to make
anhydrous copper acetate, add a saturated ethanolic solution of CuCl2 to a saturated ethanolic solution of potassium acetate until precipitation
ceases, filter, and distill off ethanol to yield mostly copper acetate containing a little potassium chloride. Hopefully ethanol's affinity for water
will help ensure the resulting solids are free of water, but all of this must be carried out while carefully avoiding atmospheric moisture, in freshly
dried ethanol, it would seem.
Potassium acetate appears to be a little easier to dry than sodium acetate: the sesquihydrate converts to a semihydrate at 41 C, according to
Wikipedia. I don't know when the semihydrate loses water, though.
And CuCl2 isn't too hard to dry, right?
http://www.sciencemadness.org/talk/viewthread.php?tid=15485&...
[Edited on 24-7-2017 by clearly_not_atara]
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
I'm not sure about that atara: copper chloride is strong enough an oxidant to oxidize ethanol to acetaldehyde (a quick back-of-the-envelope
calculation shows that the reaction is slightly favorable even without accounting for the solvation energy of HCl in ethanol). Copper acetate is
probably similar, and so I'd probably expect that distilling off the ethanol would induce copper (I) contamination, or at the very least some water of
hydration due to atmospheric oxidation. Maybe vacuum distillation? Still, I'd expect some reduction of the product by solvent.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
That's a problem for sure.
Interesting. I think vacuum distillation is probably necessary as you suggest. But if CuCl2 or Cu(OAc)2 were a good oxidant for ethanol we could take
this to the acetaldehyde thread 
I found the attached paper which mentions potassium acetate in acetonitrile for the ring-opening of an epoxide (page 1, example 3). However, I haven't
found a ref for the solubility of KOAc in acetonitrile. I believe both copper chloride and acetate are soluble in MeCN. KCl certainly is not. This
paper also mentions using "2 equivalents of anhydrous potassium acetate in acetonitrile" for something although I haven't got it:
http://pubs.acs.org/doi/abs/10.1021/jo400774u?journalCode=jo...
So if we can confirm solubility of KOAc in acetonitrile that could be a winner, although now it requires acetonitrile again, but this might be easier
than going through acetyl chloride by gassing HCl and it allows you to reuse both MeCN and copper. Conveniently, acetonitrile is much less
hygoroscopic than anhydrous ethanol.
Formamide (not dimethylformamide) also apparently dissolves KOAc according to Wikipedia, although I'm not familiar with formamide as a solvent (it
apparently has some other uses in inorganic chemistry?). Acetamide might also work?
Paper attached.
Attachment: orellana2014.pdf (168kB)
This file has been downloaded 650 times
EDIT: according to this, CuCl (cuprous) is soluble in acetonitrile:
http://alpha.chem.umb.edu/chemistry/ch371/CH371_Information/...
this could be useful since CuOAc likely has no hygoroscopicity at all but may still provide the anhydride when heated.
[Edited on 25-7-2017 by clearly_not_atara]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
(Too late to edit above post)
After investigating, detailed references to anhydrous KOAc in MeCN always use 18-crown-6. It turns out this can do some cool things -- including the
Lossen rearrangement, amazingly -- but it won't precipitate KCl like we want it to, and crown is expensive.
But copper (i) compounds dissolve in acetonitrile or in solvent mixtures containing acetonitrile. Furthermore they are much less likely to oxidize
ethanol. So the new version is to dissolve CuCl in azeotropic acetonitrile/ethanol and add saturated KOAc in ethanol. It should still be possible to
recover and reuse solvents. And the solutions can be dried over an insoluble salt like MgSO4 which helps I think.
The trouble is now we have copper (I) acetate. But as this salt is more similar to the silver salt, that doesn't seem like such a bad thing. Oxidation
of the product is now unlikely. Wiley says that heating copper (II) acetate in vacuo to 220 C gives copper (I) acetate, which makes it seem like
acetate gets oxidized. Copper (I) acetate might actually work better. Wiley says it decomposes at 285 C but not into what or under what conditions (O2
atmosphere?)
The last thing is you need a dry atmosphere as well for the actual distillation, because water initiates autocatalytic decomposition of the product
and must be rigorously excluded. Maybe a drying tube will work but you probably want a nitrogen tank or similar.
[Edited on 27-7-2017 by clearly_not_atara]
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
symetrical anhydrides with sulfated Zirconium
Dawt,yes this really works,
the one thing to know is the amount of PEG-1000 to use is based on it's molwt = 62g/mol-1.
You cannot cheat and start with inexpensive Zirconium Oxide. ZrO2. The crystal lattice must be the calcined-formed tetragonal. There is a manifold
increase in surface area hence greater catalytic ability. Reactions without the tetragonal form yield ZIP.
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
sulfated Zirconia is so easy leaves halogen Rx in the dust
Quote: Originally posted by dawt  | Seems nobody has mentioned this paper before. They claim to have prepared AA from acetic acid in a 90 % yield after 2 h at 40 °C using a PTC and
zirconia catalyst. Sounds too god to be true, eh? The fact that it was published in some Ethiopian journal doesn't raise my confidence, but it looks
cheap enough for me to give it a shot some time next month when I have money 
| Quote: |
Preparation of sulfated zirconia
The sulfated zirconia catalyst was prepared by equilibrium adsorption of sulfate species on the surface of hydrous zirconium oxide samples. The
hydrous zirconia catalysts were prepared by precipitation method using ZrOCl2.8 H2O and liquid ammonia solutions. Required
amount of zirconyl chloride solution was added dropwise to deionised water. The pH was maintained at 10.0 by controlled addition of ammonia solution
to the reaction mixture. The precipitated solution was stirred for 16 h at 35 °C followed by filtration and washing with double distilled water until
free from chlorine ions. The hydroxide precipitate were subsequently dried overnight at 100 °C and calcined at 250 °C for 24 h. To prepare sulfated
ZrO2 catalyst, a portion of the obtained hydrous zirconia sample was ground to fine powder and immersed in 1 M H2SO4
solution. Excess water was evaporated on a water-bath and the resulting sample was oven-dried at 120 °C for 12 h and calcined at 500 °C for 4 h in
air atmosphere and stored in vacuum desiccator.
General procedure for the preparation of symmetrical carboxylic anhydrides
To a stirred solution of carboxylic acid (10 mmol) and PEG-1000 (5 mmol) was added SO42-/ZrO2 (3 mmol) at room
temperature and stirring was continued at 40 °C for the appropriate time. After completion of the reaction, as indicated by HPLC, the product was
extracted with methylene chloride (3 × 5 mL). The combined organic layer was dried over anhydrous Na2SO4 and then filtered over
a pad of flash silica gel. Removal of the solvents in vacuo furnished the corresponding pure symmetrical carboxylic anhydrides. Fresh substrates were
then recharged to the recovered catalyst system and then recycled under identical reaction conditions.
|
Ref:
Lin Hu, Y., E Zhao, X. & Lu, M., 2011. Efficient and convenient synthesis of symmetrical carboxylic anhydrides from carboxylic acids with sulfated
zirconia by phase transfer catalysis. Bull. Chem. Soc. Eth., 25(2). Available at: http://dx.doi.org/10.4314/bcse.v25i2.65900.
[Edited on 9-5-2016 by dawt]
[Edited on 9-5-2016 by dawt]
[Edited on 9-5-2016 by dawt] |
hey Dawt, this does work and works well
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
Trying to get this posted in the right place.
Dawt, the sulfated Zirconia reactions works very well and it can be reused 5X.
you need to know what the weight of the PEG-1000 used is and that's simple.
Amazingly simple and no where near the odor or toxicity of these other idiotic halogen reaction. Did this in an apartment next to the managers unit
without any problems. Amazing.
[Edited on 7/24/2017 by spankyetal]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
How did you separate AA from PEG-1000?
|
|
|
| Pages:
1
..
31
32
33
34
35
..
43 |