| Pages:
1
..
32
33
34
35
36
..
43 |
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
Zirconium sulfate or sulfated Zirconia ?
Quote: Originally posted by clearly_not_atara  | byko3y: Noted. Although I continue to insist that the differences in reactivity between SCl2 and S2Cl2 can be considered analogous to C2H4Cl2 vs
CH2Cl2. SCl2 has a much smaller dipole moment because the two S-Cl bonds are in opposing directions. Likewise SOCl2 and COCl2 have large dipole
moments because the C=O and S=O bonds are highly polarized.
It seems like the intent was to make anhydrous zirconium (IV) sulfate, Zr(SO4)2, which is both a dehydrating agent and a Lewis acid. Zirconium (IV)
and titanium (IV) both like to form covalent-ish bonds with chlorine, nitrogen and oxygen, and TiCl4 is volatile. However, note that while it is a
metal salt, it's not exactly easy:
". Excess water was evaporated on a water-bath and the resulting sample was oven-dried at 120 °C for 12 h and calcined at 500 °C for 4 h in air
atmosphere and stored in vacuum desiccator."
I doubt that anhydrous zirconium sulfate can be made by an "easy" method. It's likely that simply adding zirconium to anhydrous sulfuric acid will
result in passivation. In this case the half-reaction comprises 3 moles of Zr(SO4)2 reacting with 5 moles (acid / 2) of water.
http://www.sciencedirect.com/science/article/pii/00406031938...
Notably, the method as reported is consistent with this analysis of the thermodynamics of zirconium sulfate, since Zr(SO4)2 begins to decompose just
above 500 C at 540 C. The half-reactions are Zr(SO4)2 + H2O >> Zr(SO4)2*H2O, Zr(SO4)2*H2O + H2O >> Zr(SO4)2*2H2O, and the one where I know
the energetics, 2 AcOH + ~90 kJ/mol >> Ac2O + H2O. So the heat of hydration of Zr(SO4)2 would have to be at least 90 kJ/[mol H2O], probably
higher as entropy decreases.
[Edited on 15-5-2016 by clearly_not_atara] |
Are you possibly mixing up Zirconium sulfate with sulfated Zirconia? Two entirely different compounds. Catalytic properties of sulfated Zirconia to
make symmetrical anhydrides is based upon the tetragonal form of that substance created in an oven at 600 C. Concentrated sulfuric acid does interact
with Zirconia.
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
Just like the post says, the product was extracted with methylene chloride (3 × 5 mL).
simple and sweet.
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
 so I said to myself- self! what am I doing here? gotta run. Gotta go check the
Pr. anhyd. reaction. so I said to myself- self! what am I doing here? gotta run. Gotta go check the
Pr. anhyd. reaction.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Sulfated Zirconia method is bullshit,
Anhydride make ester in reaction of Alcohol or Diol even at room temperature
We discus about this method some pages ago
[Edited on 30-7-2017 by Waffles SS]
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by madscientist  | | I'm theorizing that concentrated acetic acid could be dehydrated to acetic anhydride by mixing with concentrated sulfuric acid; then heating, and
condensing the vapors, yielding acetic anhydride. Any comments or additional ideas? |
I came across a published route to symmetrical anhydrides of carboxylic acids via hot conc. H2SO4. The acid is sprayed into the hot sulfuric acid at
elevated temps and the vapors (anhydrides) are condensed. Many reactions you'll find may be feasible for lab scale but due to safety and economic
concerns never make it to the $$ industrial scale. Seems the future of "green chemistry" - I shudder using the words- is focused on nano sized silica
or Zirconium catalysts. Remarkable non-solvent reactions yields of >94%
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
dehydration with hot sulfuric acid
Quote: Originally posted by Coen  | I am sorry to dissapoint you, but it is not possible to dehydrate acetic acid to Ac2O.
Some time ago I was also working on the synthesis of this compound, but when I got from a friend about 1,5 L Ac2O, I stopped with that .
I strongly discourage you to do something with the route using ketene. Ketene is very dangerous. The setup is very difficult to realise. The procedure
gives very low yields.
The whole process is only used in industry, and you can not always transform such syntheses to lab-scale.
I've did thought out another route using acetaldehyde which could be realised fairly easy. But you do have to make/buy acetaldehyde first for that.
Acetaldehyde can be made by leading acetylene through a solution of a Hg2+ salt and then condensing it. |
pulled as repetative
[Edited on 7/24/2017 by spankyetal]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Waffles SS  | Sulfated Zirconia method is bullshit,
Anhydride make ester in reaction of Alcohol or Diol even at room temperature
We discus about this method some pages ago |
This was my expectation as well, but:
* owing to the chain length of PEG-1000 (~25 ethers) it's possible that, even with solvolysis, not much of the AA was consumed
* he claims to have succeeded (!) and experiment > theory generally
[Edited on 1-8-2017 by clearly_not_atara]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
*Even if not much of the Ac2O was consumed , all reactant and product are soluble in CH2Cl2 and this mean you can not separate Ac2O by this solvent.
after dissolving Ac2O in CH2Cl2 it will continue to react with remaining PEG
This is difficult to believe articles in Ethiopian journal
Quote: Originally posted by madscientist  | | I'm theorizing that concentrated acetic acid could be dehydrated to acetic anhydride by mixing with concentrated sulfuric acid; then heating, and
condensing the vapors, yielding acetic anhydride. Any comments or additional ideas? |
This is possible below -5C
Gilbert, E. E.Chemical Reviews (Washington, DC, United States), 1962 , vol. 62, p. 549 - 589
[Edited on 2-8-2017 by Waffles SS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
^^^Interesting. Since it relies on the volatility of Ac2O, maybe gassing the H2SO4 with HCl first would yield AcCl, and, since this has a much higher
vapor pressure, it would be more practical. Something tells me that you're going to get extremely slow evaporation of Ac2O at -5 C...
Of course, HF would do even better, making acetyl fluoride (bp 21 C), but there are safety issues in dealing with fluorides.
[Edited on 2-8-2017 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Ok, so how about this plan:
1. Distill ammonium acetate through an 80-100 C column to prepare acetonitrile.
2. Gas acetonitrile with hydrogen chloride to prepare acetyl chloride.
3. React acetyl chloride with sodium acetate to prepare acetic anhydride.
The column could be a simple Liebig condenser circulating boiling water or a low temperature tube furnace in vertical position around a glass tube. It
would be kept slightly above the melting point of acetamide to keep the apparatus from getting clogged.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | Distill ammonium acetate through an 80-100 C column to prepare acetonitrile. |
Acetonitrile is made by refluxing acetamide over acids. The decomposition of ammonium acetate proceeds through the diacetate which decomposes
to acetamide + acetic acid + water. So first ammonia is lost leaving ammonium diacetate, and then this decomposes to release acetic acid and water,
leaving acetamide in the condensed phase. The Orgsyn procedure for acetamide uses 50 moles of acetic acid to 23.5 moles of ammonia:
http://www.orgsyn.org/demo.aspx?prep=CV1P0003
"In a 5-l. flask is placed 3 kg. (2860 cc., 50.0 moles) of glacial acetic acid and to this is added a weight of ammonium carbonate corresponding
to 400 g. (23.5 moles) of ammonia (Note 1). The flask is fitted with a one-hole stopper holding an efficient fractionating column 90 cm. long with
condenser and receiver. An air condenser 150–200 cm. long may be employed. The mixture in the flask is heated to gentle boiling and the flame so
regulated that the rate of distillation does not exceed 180 cc. per hour. The distillation is continued in this way for eight to ten hours, until the
temperature at the head of the column reaches 110°."
It is ideal to produce acetamide and then reflux this, since the initial release of ammonia and acetic acid is likely to be obnoxious if it flows
through the vessel which is meant to capture acetonitrile.
In chemplayer's synthesis of acetyl chloride I believe he used equal amounts of acetic acid and acetonitrile. Since acetic acid is much easier to
produce I think it wouldn't hurt to use it in excess; this may improve the yield relative to acetonitrile.
[Edited on 3-8-2017 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Do you have a source indicating that the decomposition of ammonium acetate proceeds through the diacetate? I ran across a document that stated that
recently, but it was published before the American Civil War, so I considered it apocryphal. Also, the reference that you cite (which is a good
reference) directs making ammonium acetate in situ and distilling it through a long column (essentially a reflux) but doesn't mention any ammonia in
the distillate even though there is only a slight excess of acetic acid.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | Also, the reference that you cite (which is a good reference) directs making ammonium acetate in situ and distilling it through a long column
(essentially a reflux) but doesn't mention any ammonia in the distillate even though there is only a slight excess of acetic acid.
|
A slight excess? There are 50 moles of acetic acid and 23.5 moles of ammonia. Not 23.5 moles of ammonium carbonate:
"a weight of ammonium carbonate corresponding to 400 g. (23.5 moles) of ammonia"
The wording's awkward, but it states a 2:1 ratio of acetic acid to ammonia.
See also the attached graph of the decomposition of NH4OAc. You can see two peaks on the differential thermal analysis (DTA) curve which shows a phase
transition before complete transition indicating a sudden peak of heat capacity, but the gravimetric curve (DTG/TG) only shows the final decomposition
peak. This is consistent with the loss of ammonia since only a small fraction of mass is lost but the composition of the salt changes. And while the
paper (also attached) does not mention what the intermediate product is, it does state the presence of an intermediate product.
http://en.wikipedia.org/wiki/Thermal_analysis
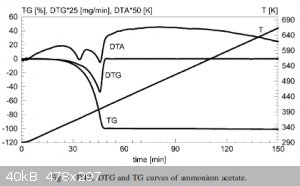
Attachment: olszakhumienik2001.pdf (105kB)
This file has been downloaded 954 times
[Edited on 3-8-2017 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It states a slightly larger than 2:1 ratio of acetic acid to ammonia 
It does look like thermal decomposition of ammonium acetate is minimal below 50 C at atmospheric pressure.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
For making AcCl from Acetonitrile The only reference that i can find is this(i dont have access to read it)
Perrot
Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1938 , vol. 206, p. 1576
This is difficult for me to believe this method,even chemplayer is not sure about final product and he didnt analysis it so far(just measure boiling
point and reaction with Sodium acetate is not enough)
[Edited on 3-8-2017 by Waffles SS]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Here is is on Google Books: https://books.google.com/books?id=Z9RFAQAAMAAJ&pg=PA1155...
I hadn't actually read this paper until just now, but it looks as though you could chlorinate any carboxylic acid in acetonitrile with this method.
(I should clarify that they couldn't get it to work with formic acid, and obviously some functional groups could cause a problem.)
[Edited on 3-8-2017 by JJay]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It doesn't seem like a particularly outlandish mechanism to me. First acetonitrile reacts with one equivalent of HCl to the imidoyl chloride. Then the
imine nitrogen is protonated by a second molecule of HCl to the iminium chloride, and this attacks acetic acid to give acetic anhydride monoiminium,
which reacts with the chloride ion to release acetyl chloride and acetamide.
Plus it might not have been fully characterized but how many possible products are there with that BP?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
This is difficult to guess about product with this BP.He should do more scientific method for analysis the product(IR-Gc-Ms,..)
Another reason that i am doubtful about this method is reaxys serach of AcCl with acetamide.(In most case N-acetylacetamide is product)
Attachment: AcCl+AcNH2.pdf (121kB)
This file has been downloaded 886 times
[Edited on 3-8-2017 by Waffles SS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Nearly all of those reactions involve basic conditions, though (pyridine, collidine, lutidine...). And most of them are in aprotic solvents. But just
look at what happens when acetic acid is the solvent: in one case you get the imino-anhydride, and in another, some kind of ketene aminal.
There is one reaction with FeCl2 in AcOH that gives an imide. But the use of a catalyst implies this product is not typical, doesn't it?
[Edited on 3-8-2017 by clearly_not_atara]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
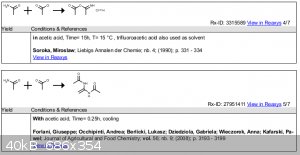
| Quote: |
Acetamide (0.2 mol) was dissolved in acetic acid (40 mL)and cooled in an ice bath. Then acetyl chloride (0.1 mol) was addedwith cooling, and the
formation of a crystalline byproduct was observed.
Forlani, Giuseppe; Occhipinti, Andrea; Berlicki, Lukasz; Dziedziola, Gabriela; Wieczorek, Anna; Kafarski,
Pawel;Journal of Agricultural and Food Chemistry; vol. 56; nb. 9; (2008); p. 3193 - 3199 |
| Quote: |
But just look at what happens when acetic acid is the solvent: in one case you get the imino-anhydride, and in another, some kind of ketene amina
|
Thanks but Please provide reference?
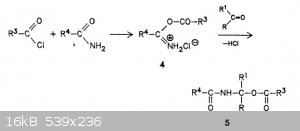
| Quote: |
The primary product of the attack of an acyl chloride on an amide is the
0-acylisoamide hydrochloride 4. Since all of the amide is trapped into
a “complex” by acetyl chloride there is no “free” amide available
for the reaction with aldehyde, hence, N,N-alkylidenebisamides should not exist in the reaction mixture
Soroka, Miroslaw; Liebigs Annalen der Chemie; nb. 4; (1990); p. 331 - 334
|
| Quote: |
First acetonitrile reacts with one equivalent of HCl to the imidoyl chloride. Then the imine nitrogen is protonated by a second molecule of HCl to
the iminium chloride, and this attacks acetic acid to give acetic anhydride monoiminium, which reacts with the chloride ion to release acetyl chloride
and acetamide.
|
Imidoyl chloride can be prepared by treating a monosubstituted carboxylic acid amide with carbonyl chloride or halogenating agents.I dont think HCl is
enough powerful for this.Please provide reference for your idea
Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; pp. 55–112.
[Edited on 4-8-2017 by Waffles SS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Nitriles react with HCl but the reaction does not stop at an imidoyl chloride:
https://www.researchgate.net/publication/244701085_Studies_o...
However, as the fragmentation of imidoyl chloride to nitrile + HCl is well known it is not too far gone for me to assume the reaction reverses to a
small extent at equilibrium.
The requisite "dehydrating potential" comes from the energy of hydration of acetonitrile; HCl is just a catalytic acid.
Hf 298 (an unreliable estimator, but if one side is way bigger the reaction favors that sie)
Acetonitrile: -40 kJ/mol
HCl: -92 kJ/mol
Acetamide: -310 kJ/mol
Acetic acid: -486 kJ/mol
Acetyl chloride: -274 kJ/mol
40 + 92 + 486 = 618
310 + 274 = 584
The left side wins very slightly, but when you take into account that acetamide (pKa of conjugate acid = -0.5) is much more basic than acetonitrile
(conjugate acid is strong in water), acetyl chloride formation looks preferable. I don't have the Hf values for acetamidium and acetonitrilium in
acetic acid, though :p
| Quote: | | Most nitriles having α-hydrogen react with HCl to give N-(α-chloroalkenyl)alkylamidine hydrochlorides (3). |
This is consistent with formation and subsequent dimerization of imidoyl chlorides (attached). The possibility of such dimerization supports my
hypothesis that using an excess of acetic acid will improve yields, IMO.
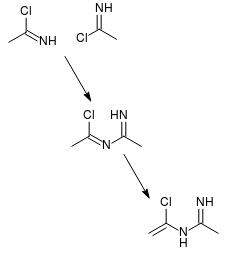
[Edited on 4-8-2017 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Is the dimerization reversible?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I think probably not. However, upon further consideration, it's possible the dimer is actually the active chlorinating agent. If you add HCl to the
dimer, you get 1,1-dichloro-N-(ethylimino)ethylamine, which is structurally very similar to dichloromethyl methyl ether and also to dichloroacetic
acid, both of which are known chlorinating reagents for the AcOH -> AcCl transformation.
I can't tell what pathway it is, although the dimer seems a somewhat more plausible chlorinating agent on energetic grounds. One way to know would be
to try reacting GAA with pre-formed N-(1-chlorovinyl)acetamidine and HCl and compare the acetyl chloride yield to that obtained with acetonitrile.
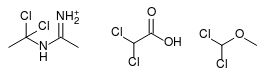
[Edited on 12-8-2017 by clearly_not_atara]
|
|
|
spankyetal
Harmless

Posts: 23
Registered: 24-7-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | Quote: Originally posted by Waffles SS  | Sulfated Zirconia method is bullshit,
Anhydride make ester in reaction of Alcohol or Diol even at room temperature
We discus about this method some pages ago |
This was my expectation as well, but:
* owing to the chain length of PEG-1000 (~25 ethers) it's possible that, even with solvolysis, not much of the AA was consumed
* he claims to have succeeded (!) and experiment > theory generally
[Edited on 1-8-2017 by clearly_not_atara] |
Hate to burst your bubble but I have gobs of anhydrides created from their corresponding acids. Sulfated Zirconia is NOT Zirconium Sulfate.
i.e. - precipitated Zirconium hydroxide is dried, sulfated with 0.1 M solution of conc. sulfuric acid, calcined 4 hrs at 550 Centigrade. 2 moles of
the acid yield 1 mole of the anhydride. Bada-bing bada-boom! Room Temp.
Nothing happens without the correct molar amount of PEG-1000. More P. Anhydride than ever imagined- etc. - disclaimer, I have violated no laws within
the jurisdiction or Territories of the United States.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
@spankyetal
I understand WHY you're concerned, but disclaimers just make people wonder what you're talking about and draw attention to you.
[Edited on 11-9-2017 by SWIM]
|
|
|
| Pages:
1
..
32
33
34
35
36
..
43 |