| Pages:
1
..
33
34
35
36
37
..
43 |
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
I haven't read all the proposers in this thread, quite a few, but I want to somewhat awkward look at what would potentially happen if one added GAA,
dry if possible, to a flask, say 100ml, and to that, one adds say, fifty ml of dry toluene, and also 10ml of 80% sulphuric acid, and DS conditions,
and very importantly prevent the moisture from entering via the condenser sitting on the DS trap.
So the way it looks to me is one molecule would react with a secondary molecule via some nucleophilic addition, 1,2 addition, then followed by a 1,2
elimination, which creates the bad guy as H2O that is a good nucleophile, thus causing the anhydride to hydrolyse, I think is the word, but in short,
being reverted back to the two acetic adds, so what about the azeotrope beginning to be formed by the release of these water molecules, and with DS
trap, would theoretically result in toluene water azeotrope, bp at 84.1C, but as we know when that chills post hitting the condenser, the water makes
its way to the bottom of the toluene layer. So the other players are
acetic acid b.p.118-119C
The anhydride 139.8C
Toluene and water azeotrope being 84.1C
This bunny wont play with list one anythings, well most things, but this thing, nope, but people know people, and its been stated to work. After
refluxing where no more increase of water is created, then the trap empties, then gets filled up again with the expected, as well as toluene, as
toluene is so like benzene, so if benzene is clearly known to be in solution together with the anhydride formed, so toluene would also. The person who
spoke up said that he'd worked out the theoretical amount of water eliminated and when that was obtained, although now being generated much more
slowly, he factored it into the solvents must have got water, especially the only species left are the supposed to be Tol, and the anhydride, then he
realised he hadn't plugged up the condenser, with a dry dry salt, a few more mls after doing so, then nada, for a good 40minute rate. Please don't
jump on me to hard, Im only a passing wind, potentially dreaming of such ease, but its experiment worthy, and of course this has been thrashed to
pieces, my apologies etc.
One could pretty simply reduce the needed amount of toluene to bind somewhat with the H2O from this addition reaction, but given if toluene is in the
pot, and water is being formed, then its dancing shoes till not. Anyway, its all a learning curb for here, and I feel it may well have merit.
Cheers folks
[Edited on 21-10-2017 by Babs]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If the Dean Stark is pulling the water out you're going to start getting sulfonation of the toluene at some point as the acid concentration goes up.
I suspect sooner rather than later.
This ought to be able to happen before it gets dry enough for Acetic anhydride formation as you can sulfonate toluene without an especially dry
environment (no drying tube, no need for 100% sulfuric acid).
I Have no idea if mixed toluenesulfonate isomers would be worse or better than sulfuric acid for trying to make acetic anhydride(I suspect worse), but
I'm pretty sure they would be generated in your reaction mixture.
[Edited on 21-10-2017 by SWIM]
Comment on below post: Yeah I totally missed that, but I get it now, thanks.
[Edited on 22-10-2017 by SWIM]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You can't dry acetic acid with sulfuric acid. Even ignoring sulfonation, the bp of water increases when it's mixed with H2SO4, so the toluene/water
azeotrope will not form.
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
I trust the source, so Im saying it does. I also see that 10ml of 80%, is excessive, as it only requires one proton to promote what does occur, but
quickly gets reversed due to the nucleophilic eliminated H20. IF that gets mopped up, over a long function of time, then surely one would have the
cream. Sulfonation, I am not sure.
Whether the azeotrope can also include H30+ and anions, albeit ever so minimally, I am not sure, my guess is yes.
Google searching- can an azeotropic mixture ever include anions and protons. The answer is yes. Hydronium nitrate found in an azeotropic mixture
between water and nitric acids. So if this is indeed so, the more acidic it gets, the more these ions are forced into bonding amidst the azeotropic.
Im stating this, as Im not interested in being right, but it appears this could be. I cannot link, and the URL is to long to just copy paste, but its
an interesting read. Re-Google books.
"Biophysical and Structural Aspects of Bioenergetics"
[Edited on 22-10-2017 by Babs]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The constant boiling mixture of nitric acid and water is a negative azeotrope, i.e. it has a higher boiling point than either of its components. This
is the reverse of a typical azeotrope but because it has a certain useful property (it can be distilled) we call it "azeotropic". A mixture of water
and HCl has a higher boiling point than either and yet HCl does not intersect water at all in their liquid ranges.
Point is, your source is wrong. The presence of acids lowers the vapor pressure of H2O. Azeotropes form because the lower cohesion in a mixture vs the
pure substance leads to higher vapor pressures; acids cause higher cohesive forces because of ionization. Thus constant boiling mixtures of HCl, HBr,
HNO3 with H2O all boil above 100. This is the opposite of what happens with toluene. You are being misled by the flexibility of the word "azeotrope":
the nitric acid/water azeotrope is the OPPOSITE of the toluene/water azeotrope.
It's mildly annoying to watch people spend time on things that obviously won't work. But only mildly. Ho hum.
Sulfuric acid will never dehydrate acetic acid. The equilibrium:
2 [AcOH] <> [H2O] + [Ac2O]
leans so far to the left that the concentration ratio of [H2O]/[AcOH] will always overpower the greater volatility of H2O vs AcOH. As such AcOH boils
away faster when the mixture is heated.
If something so trivial worked, this thread would be entirely unnecessary.
[Edited on 22-10-2017 by clearly_not_atara]
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
quote,
“Examples of this geometry for hydrogen bonds of water and hydronium ions are to be found in azeotropic mixtures of “WATER WITH ALCOHOL’ which
has a lower bp, I believe. it goes on to include water with strong(mineral) acids, and used the one that, is positive, or has a higher bp, as you have
implied, as one example. The point I see it makes is azeotropic mixtures appear to, or can, include hydronium ions, and anions. Which is a big point,
IMO
As far as Le Chatelierl's principals deal goes, yes its so far over to the left, but you are adamant that anything on the right could ever be moved,
and Im not buying it. I fully believe an azeotropic mixture gets formed and that my knuckle headed friend who wrote me this, aint lying
I wont go on and I know this is flying in the face of years of questing this, and I can sense frustration, and i definitely don't want to waste
peoples time.
Whenever I see something that appears to be so ridiculously challenged, my head goes to the Helicopter virus, the actual cause of stomach ulcers, but
hey, this is inquiry, exploring, daring to state what I doubt would be taken as plausible, but Im not backing down. Sorry to be an annoying noob.
I am not sure what you mean by boils away faster, as the lowest bp would be the water toluene azeotropic mixture, that you wont consider, and Im
painfully stating otherwise. My tone is brotherly, but sometimes, with things that appear huge hurdles, a simple method isn't going to be considered.
Hiding in plain sight. Now Ive probably pissed you of even more by stating that, but I believe their is some truth to that, and see it app here.
Cheers mate.
[Edited on 22-10-2017 by Babs]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | It's mildly annoying to watch people spend time on things that obviously won't work. But only mildly. Ho hum |
This vale of minor irritations... LOL!
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by hissingnoise  | | Quote: | | It's mildly annoying to watch people spend time on things that obviously won't work. But only mildly. Ho hum |
This vale of minor irritations... LOL!
|
Exactly,
We are going backward in chemistry basics every day in this topic
[Edited on 22-10-2017 by Waffles SS]
|
|
|
CaptainMolo
Harmless

Posts: 30
Registered: 24-8-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Babs  |
I cannot link, and the URL is to long to just copy paste, but its an interesting read. Re-Google books.
"Biophysical and Structural Aspects of Bioenergetics" |
I tried to find this on google books to no avail, is there an author or an ISBN number I can use to find this? It sounded like an interesting read.
Also < / lurk> :p
[Edited on 22-10-2017 by CaptainMolo]
Only two things are infinite, the universe and human stupidity, and I'm not sure about the former. - Albert Einstein
Find me on BitChute, YouTube and MINDS as "Full
Modern Alchemist" |
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | I fully believe an azeotropic mixture gets formed and that my knuckle headed friend who wrote me this, aint lying |
Vapor pressure is continuous. The lower bp component only leaves faster if its vapor pressure is higher. And the vapor pressure of a component
decreases with lower concentration.
http://en.wikipedia.org/wiki/Vapor_pressure
Your "friend" generalized from an inaccurate understanding of how chemical equilibriums work. At any given time, in a mixture of liquids, all
of the liquids are evaporating. Which liquid forms a larger component in the vapor depends on both concentration and volatility of the component: the
evaporate from a mixture of 99.99% AcOH and 0.01% H2O is primarily composed of AcOH.
| Quote: | | My tone is brotherly, but sometimes, with things that appear huge hurdles, a simple method isn't going to be considered. Hiding in plain sight
|
You know what else is hiding in plain sight? The reports from other people who have tried such methods that don't work, 35 pages of them in fact.
They're in this thread, which you have not read. Dehydration of acetic acid with acids wasn't "not considered", it was the first thing anyone tried.
And it doesn't work. Fancy that. You want to know just how much it was considered? It was the very first post in the thread!
https://www.sciencemadness.org/whisper/viewthread.php?tid=9#...
How could you know what was and wasn't tried if you don't look? SO3 doesn't work, Na2S2O7 doesn't work, P2O5 barely works, HPO3 doesn't work, and
H2SO4 obviously doesn't work. Toluene is a joke.
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&...
| Quote: | | Whenever I see something that appears to be so ridiculously challenged, my head goes to the Helicopter virus, the actual cause of stomach ulcers, but
hey, this is inquiry, exploring, daring to state what I doubt would be taken as plausible, but Im not backing down. Sorry to be an annoying noob.
|
Backing down is a sign that you're the sort of person who is capable of learning, "not backing down" is the sign of someone who will never listen to
criticism and slowly develop from an annoying noob to an incessant gadfly who everyone has learned to tune out. Sorry to be a condescending prick, but
your "brotherly tone" is not actually helping to move the discussion forward, it's just a shield so that you can play the victim when people call you
out on your crap.
[Edited on 23-10-2017 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
I haven't a problem being wrong, i used to think I knew a fair bit of chemistry, but in time, the thing I learnt was how huge this Art is and how
little I did know. I don't mind at all appearing foolish but that said, I still won't backdown. I could, can, but at the risk of looking even more
retarded, can't. That said, I can't present a spectral analysis of what knucklehead sorted, only the fact we are closer than close and as such he
won't lie. I'll have a look at the posts you presented, but maybe this needed excess H+, but that said. If the moderators here believe I am
deliberately being obstanant, and what I have presented is so obviously daft, including my responses that present a degree of argument to the initial
responses as to why this is bs, etc, then I'll gladly delete the first post hence gone. I am assuming that's how it works, if not maybe the moderators
can wipe my so called bs findings.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The moderators could, but won't do what you suggest, and you know that . Again you use something to hide behind, putting your shit in someone else's
hand because that is how you try to look "right". Nice try but won't work
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
Well I don't in fact, I think Ive seen or heard of posts being moved to beginners or something but you call it hiding, I merely mentioned it as an
option. See I don't see what Im suggesting as anything but an interesting manner, in the context of this thread, and when checking out the first post,
I do not see that this deal has been explored at all. Adding acid to acetic, reflux and distill, is all I saw, but that aside, Im done, and thsnkyou
for the critiques, suggestions etc.
Cheers.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
@Babs: People are telling you it won't work. If you don't believe them, feel free to try to prove them wrong with actual experimentation. I can't say
that reputation doesn't matter at all on ScienceMadness, but it doesn't matter that much; if you are discussing things scientifically with a solid
factual background, people will notice and respect that.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
With regard to the claim that "anything can happen" - in chemistry, my friend, that is certainly true. Just on the last page Waffles SS and I
were arguing about the plausibility of the claimed synthesis of acetyl chloride using acetonitrile and hydrogen chloride as the combined chlorinating
agent. Neither of us could have predicted the eventual discovery of a dimer of acetonitrile formed by HCl, N-(alpha-chlorovinyl)acetamidine. The
existence of this dimer threw off all of our previous arguments about the reactivity of the compounds.
However, you have not claimed any new chemical transformation. You have, instead, talked only about the thermodynamic properties of
the substances you are describing. Such as azeotrope formation and so on. But thermodynamics is not chemistry: it does not teem with surprises. You
cannot cheat at thermodynamics with catalysts. So H2SO4 does not shift the equilibrium, and to the extent that it does, it adheres to water molecules
and prevents them from boiling away. If you heat dilute sulfuric acid to 250 C, it will still contain water. And toluene lowers the bp of water but
crucially does not reduce the vapor pressure (!!) any more than it does that of AcOH. And generally solvents which would have such a large
differential effect on vapor pressure do not exist and have never been described. The vapor pressure of water is too low to effect this transformation
by several orders of magnitude so a 15 C azeotrope is pissing into a canyon.
This is why I can know that you are wrong. Also, if you read the thread, you will learn that treating AcOH with strong acids can cause dimerization
and also that your rxn system may lead to Friedel-Crafts rxns between AcOH and BnH. This may account for any observed water production or unexpected
smells. Happy wasting reagents day! (It's every day!)
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CaptainMolo  | Quote: Originally posted by Babs  |
I cannot link, and the URL is to long to just copy paste, but its an interesting read. Re-Google books.
"Biophysical and Structural Aspects of Bioenergetics" |
I tried to find this on google books to no avail, is there an author or an ISBN number I can use to find this? It sounded like an interesting read.
Also < / lurk> :p
[Edited on 22-10-2017 by CaptainMolo] |
https://books.google.com.au/books?id=5G0oDwAAQBAJ&pg=PA2...
|
|
|
Babs
Harmless

Posts: 16
Registered: 28-7-2017
Member Is Offline
Mood: No Mood
|
|
With respect to the comment that toluene lowers the boiling point, well If a mixture has a high vapour pressure it means that it will have a low
boiling point. To isolate water, and state that its vapour pressure hasn't changed, well yes, but in the context of a mixture, if the boiling point
has been lowered, then its vapour pressure surely has increased, Im probably of base, but the vapour pressure is also dependant on the mole fractions,
and that isn't just the 10ml of 80%, its overtime acetic acids carbonyl gets protenated and reacts with one that hasn't been, as in a nucleophilic 1,2
addition then elimination, and the eliminating specie is water. Sure much of it going to act itself as a nucleophile, but some surely could get caught
in the azeotropic mixture, that being toluene and water, and perhaps knucklehead needs to present the actual molar amount of H+ in the context of the
molar amount of acetic acid, be it potential to be an electrophile, or a nucleophile, which I feel is a given that this isnt a problem about forming
acetic anhydride, but suppressing the role water has in breaking this given.
Now I will shut up, and I apologise if Ive been NON Scientific, and immature by defending it so hard, without more evidentiary material.
Clearly_Not_Atari, I can understand what you are stating, and feel I get that the hold between a concentration of acid, and water perse, can indeed
have, an azeotrope, where the boiling liquid and the vapour phase there and then, cannot, not remain constant, at a particular concentration or mass
percent/fraction.
The other thing I wanted to state is, How hard have I really looked at the system I have been defending. Truth of the matter is, probably not half as
much as I would need to be, to remain so obstinate, so my bad for presenting as such. One would surely have to know that its repeatable, use what one
can, as far as evidence of what is what, and i have not done that, so I have taken some comments on board, and appreciate it. Cheers.
[Edited on 24-10-2017 by Babs]
[Edited on 24-10-2017 by Babs]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Not sure if I missed this.
Has anybody actually tried the route where acetylene is bubbled into hot (90C) acetic acid with mercuric sulfate and the reaction temp rises to, and
is maintained at, 130-140C to form ethylidine diacetate and immediately decompose it to acetic anhydride? (US425500)
[ EDIT: as pointed out below, I meant 1425500. ( I MUST learn to write things down!)]
I mean sure it's just a patent, but WafflesSS says he's done something very similar, and this does look like a nearly OTC approach other than the
mercury.
Hell, all you need is the oxide, as they form the mercuric sulfate in situ in the patent.
[Edited on 24-10-2017 by SWIM]
[Edited on 24-10-2017 by SWIM]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
FWIW, and it's not much, the correct patent is US 1680760 A ─ the words "straws", "clutching" and "at" readily come to mind.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Of the various syntheses of the anhydride, the ketene process and the reaction (POCl3+NaOAc) would appear most accessible...
Red phosphorus can be used for PCl3 which is itself oxidised by chlorate.
More here!
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by hissingnoise  | FWIW, and it's not much, the correct patent is US 1680760 A ─ the words "straws", "clutching" and "at" readily come to mind.
|
Sorry, I did screw up the patent number. I was referring to the patent numbered 1425500 granted August 8 1922.
I'm not saying your assessment is wrong because of this, but I just wanted to point out that I'm not quite that bad at remembering numbers.
There are a few differences in these patents as this one is for making acetic anhydride in one pot without acetic anhydride being called for in the
initial reaction mix. It does call for more mercuric sulfate, but they were using acetic acid at concentrations as low as 96%.
If this is a blind alley it seems several chemical engineers wandered down it in the early 20th century. (Yeah, I know, some patents are total
crap ) )
Edit: It was worth posting this to get your reference on PCl3 and route to the oxychloride. Thanks for being helpful to a novice.
[Edited on 24-10-2017 by SWIM]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
hissingnoise: if you have to chlorinate something, it makes far more sense to chlorinate sulfur than phosphorus! Sulfur is cheap, phosphorus is
illegal. And S2Cl2 works just fine. But these are both inferior to the sulfur/bromine method in practice. Or the nitrogen dioxide method, because
while NO2 is dangerous it's less dangerous than chlorine.
Also, it's worth pointing out, that S2Cl2 can be made in large quantities without having to deal with a lot of molten sulfur, and therefore, without
having to deal with a lot of hot chlorine. Once you've produced a small amount of S2Cl2, this will react readily with Cl2 gas to make SCl2, which will
react with solid sulfur to re-form more S2Cl2, which can then absorb more Cl2, etc, and friends of mine have done this in order to make lots of S2Cl2.
Additionally, sulfur is soluble in S2Cl2, so you can more than double the S2Cl2 on each iteration. This is much easier than e.g. trying to chlorinate
200 g of molten sulfur -- you can chlorinate 3g molten sulfur, and then use this to make 500g S2Cl2.
This thread really hasn't gotten anywhere since we discovered the sulfur/bromine method. The only new methods in the last ten pages or so are the
acetonitrile method and the copper/silver acetate method, but the last one is extremely unreliable. And it's frigging impossible to get people to talk
about the one method that might actually be a new, OTC, "safe" procedure for making AcCl because they all insist on retreading things that
have been posted thirty times already.
Babs: "Sure much of it going to act itself as a nucleophile, but some surely could get caught in the azeotropic mixture" -- for the last time,
there is absolutely no such thing as "getting caught" in an azeotropic mixture. Your post is attempting to justify a procedure that
does not work based on a phenomenon which does not exist. The only thing which is implied by the word "azeotrope" is that the
relative vapor pressures of two components of a mixture have the same ratio as their concentrations. The remainder of your post is not only bad
chemistry, it's atrocious English. Go to bed for fuck's sake. There is no reason for anyone to ever believe such a reaction could work; your friend is
a moron.
| Quote: | | If [acetylene] is a blind alley it seems several chemical engineers wandered down it in the early 20th century. |
It's just a really stupid alley, anything that involves the words "acetylene" and "140 C" in the same sentence should be enough to send you running in
the other direction, of course you can do that if you're a chemical ENGINEER, because you're going to spend all of your time building a huge factory
in which such conditions are actually achievable. Otherwise, you're going to burn down your house. Do you want to burn down your house?
It is possible IIRC to make vinyl acetate from acetylene and Hg(OAc)2. Vinyl acetate is useful for all sorts of other things (Heck rxn, precursor to
acetaldehyde) and IIRC it can be used to make acetic anhydride by rxn with acids. And this happens at achievable non-house-destroying temperatures and
pressures. A safe two-step method beats an explosive one-step method every day of the week.
Also I'm pretty sure acetylene has come up in this thread several times.
[Edited on 24-10-2017 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
You're welcome. The PCl3 synthesis from red P, unfortunately, requires PCl3 for initiation so you would need yellow P to start.
The ketene process requires only acetone, water and some, er, high temp. plumbing...
[Edited on 25-10-2017 by hissingnoise]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
hissingnoise: if you have to chlorinate something, it makes far more sense to chlorinate sulfur than phosphorus! Sulfur is cheap, phosphorus is
illegal. And S2Cl2 works just fine. But these are both inferior to the sulfur/bromine method in practice. Or the nitrogen dioxide method, because
while NO2 is dangerous it's less dangerous than chlorine |
Indeed c-n-a, being caught with PCl3 wouldn't be funny, but they all have pitfalls ─ and the S2Cl2 route comes with filtration headaches...
Acetaldehyde (an excuse to fuck around with acetylene hydration) oxidation has possibilities but ketene turning water to Ac2O has a biblical look to
it?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I want to put an end to the discussion of distilling acetate for preparation for acetic anhydride.
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&... - link to the original article on Zinc Acetate.
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&... - report about results by very same Waffles SS.
Now let's look inside the article - at the mass spectrum graph they recorded:
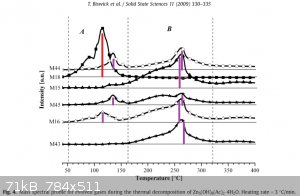
Here I made a spectrum for 260°C :
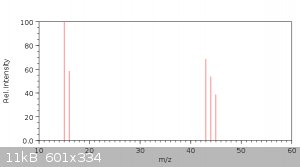
Now let's take a look at the reference mass spectrum of acetic anhydride from NIST:
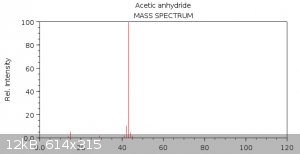
Do you see the similarity? Because I don't. How the hell could those douchebags find acetic anhydride in their spectrum - god only knows. Some more
spectrums of possible reaction products for reference (major ions in orded of decreasing intensity):
http://webbook.nist.gov/cgi/cbook.cgi?ID=C108247&Mask=20... - Acetic anhydride: 43 (faint 42)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C64197&Mask=200... - Acetic acid: 43, 45, 60 (15, 42)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C67641&Mask=200... - Acetone: 43, small 58 (42, 15)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C123422&Mask=20... - Diacetone alcohol: 43, small 58, 59 (101)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C141797&Mask=20... - Mesityl oxide: 83, 55, 98, 43, small 29, 39 (a lot of faints)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C630080&Mask=20... - Carbon monooxide: 28
http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Mask=20... - Carbon dioxide: 44 (28, 16, 12)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C7732185&Mask=2... - Water: 18, small 17
http://webbook.nist.gov/cgi/cbook.cgi?ID=C74828&Mask=200... - Methane: 16, 15, small 14, 13
http://webbook.nist.gov/cgi/cbook.cgi?ID=C74840&Mask=200... - Ethane: 28, small 27, 30, 26, 29
http://webbook.nist.gov/cgi/cbook.cgi?ID=C75070&Mask=200... - Acetaldehyde: 29, 44, 43, 15, small 42, 14
(there're similar spectrums on restek website, e.g.
http://www.restek.com/compound/view/67-64-1/Acetone - Acetone
http://www.restek.com/compound/view/123-42-2/Diacetone%20alc... - Diacetone alcohol )
Now let's try to analize the spectrum of gaseous products at 260°C: 15, 43, 16, 44, 45.
15 m/z is a methyl ion CH3+.
16 m/z is not oxygen but methane ion CH4+. Prevalence of it together with 15 m/z means methane is a major product. The fact that intensity at 15 is
higher than 16 means there are other methyl-producing substances.
44 m/z is a carbon dioxide ion CO2+. CO2 is another major product of the reaction.
There's no 18 ion meaning no water detected. That could be explained by the fact decarboxylative ketonization does not generate water but carbon
dioxide is produced instead.
45 m/z is an unknown ion. It could be acetaldehyde, but this substance has a very distinctive 29 ion which was not detected by authors. Acetic acid
has smaller ion 45, but it also has a smaller definite 60 ion, which was not detected too. My guess: 60 m/z ion was ignored for some reason (small
relative intensity?) and acetic acid is formed in substantial amounts.
43 m/z is acetyl CH3CO+. Could be derrived from any molecule with acetyl fragment. Probably that's acetone and diacetone alcohol which both have much
smaller other ions.
I'd guess the detected products were methane, CO2, acetone, diacetone alcohol, small amount of acetic acid. There's also a lot of solid products,
formed by polymerization of acetone, but those were not volatile enough to be detected.
Update: corrected ion number for acetaldehyde. Now acetic acid is the only option for 45 m/z.
[Edited on 28-2-2018 by byko3y]
|
|
|
| Pages:
1
..
33
34
35
36
37
..
43 |
|