ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
IBM RXN for Chemistry
Hi guys,
I know I have been lurking a long time but I have been busy finishing up school as well as job hunting.
The other week at ACS Boston I went to an IBM demonstration, where they are showing off their latest and greatest tool for chemistry: IBM RXN. At the
booth, they had a big touch screen showing various organic reactions, and they challenged the visitors to predict the products. Adjacent to it they
had a computer predict the products: the computer was right more frequently, and more quickly.
Basically, IBM created an AI, that learns from actual experiments either entered by other users or loaded from digital lab notebooks. Everytime
someone uses it, the better it works. It can predict products for just about any organic reaction you throw at it, and the interface is very
user-friendly and intuitive.
https://rxn.res.ibm.com/
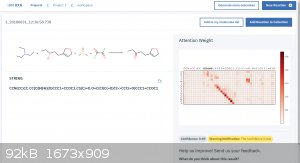
Attached is an example of it predicting the outcome of a simple Swern oxidation.
I feel like this is a great tool for the amateur as well as the professional to check their work before doing a reaction.
Have fun!
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
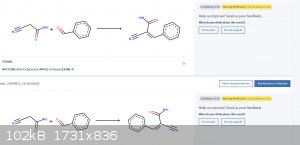
Pretty cool! I tried it for the reaction between benzaldehyde and 2-cyanoacetamide and it predicted the (main) product correctly with 49% confidence.
There are still a few problems obviously: Can't recognize that two molecules are identical (see attached picture)
I'm really looking forward to seeing how good this can get with time.
|
|
|
ELRIC
Hazard to Others
  
Posts: 244
Registered: 23-2-2015
Location: Kentucky
Member Is Offline
Mood: No Mood
|
|
I saw this on Twitter the other day and saved it to my phone. But I can’t get it to do a damn thing. I wonder if it’s not meant for mobile, or if
I’m the problem
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Wow, that is pretty cool. I plugged in a few reactions involving condensations between aldehydes, ketones, esters, etc., and for all of them it
approximated the product that I was expecting.
I wonder how well it supports the use of inorganic compounds, such as reducing agents, like NaBH4, LiAlH4, etc.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
ELRIC
Hazard to Others
  
Posts: 244
Registered: 23-2-2015
Location: Kentucky
Member Is Offline
Mood: No Mood
|
|
It looks like it can be pretty cool. But, when I click on the butane icon in the tool bar, I can’t get it to transfer over to the reaction. I was
also going to try to use sodium borohydride ( since I’ll be hitting you up soon loptr  ), but haven’t made it that far ), but haven’t made it that far
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Huh, I'll have to give that a try. I always wondered if something like that could be made. Obviously it's just an AI and doesn't have knowledge of the
underlying orbital theory, etc. - if this could be incorporated it might be even more accurate.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
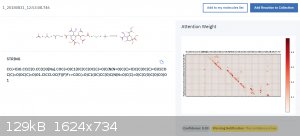
I think it does have a lot of potential... it can only get better over the years. It would be neat to look at some inorganics on it, too.
Meanwhile, I have figured out that it does not know how to do carbohydrate chemistry very well...
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|