chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Alkenes by thermal decomposition of polymers
I read about thermal decomposition of polyethylene to yield ethylene gas and of course dehydration of ethanol using sulfuric acid. However, would it
be possible to perform the same thermal decomposition on polypropylene or other alkene polymers? Any information on either depolymerization would be
great.
chemkid
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
AFAIK pyrolysis of polyolefins normally yields long list of various hydrocarbons. I did some experimenting once and impression of this short touch
was that to get anything usefull from it one has to be very skilled in separation science.
Things might be better if some catalyst is used which shortens the list of possible products or makes one prevail over all others. Does anyone know
of catalyst which could just depolymerise polyolefins on heating?
When all think alike, then no one is thinking. - Walter Lippmann
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
sulfuric acid?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I`ve distiled expanded polystyrene before when I was a kid, it make a liquid that stay liquid.
Lord knows what it was though 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It was mostly styrene, with small amouns of ethylbenzene, toluene, benzene, and likely di and trimers of styrene.
Polystyrene and the poly-acrylic acid esters, such as methyl methacrylic acid (Lucite, Plexyglas), do undergo fairly clean thermal depolymerisation.
Yields of monomers are typically in the 80 to 95 percent range.
Poly vinyl chloride and other related halo-polyethylenes mostly give HCl and unsaturated polymers, then carbon and a mixture of small hydrocarbons.
The ainyl esters, such as vinyl acetate, behave in similar fashion.
Poly-olefins generally give fairly complex mixtures of hydrocarbons, especially in the case of polyethylene.
If you concider that polyethylene is basically a chain of CH2 units, you can see that there are no 'special' positions that would favour chain
fragmentation at that point. Polystyrene, on the other hand, has a benzylic carbon at every other atom in the backbone, giving a repeating pattern of
reactive positions.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The thermolysis of polyethylene would be fairly equivalent to the so called petroleum cracking. Essencially it is about homolytic thermal cracking of C-C bonds to the apropriate radicals which recombine, disproportionate,
etc., to lower parafins. Ethene is indeed one of the products.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Well I'll be dehydrating some ethanol then. Thank you for your responses.
Chemkid
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
These are very valuable reference for anyone interested in getting valuable products from waste polymers!
http://www.docstoc.com/docs/50868604/Feedstock-Recycling-and...
http://gbhint.tripod.com/papers_5_13_02/368_Beyler_Hirschler...
Sorry, I couldn't find the more recent thread regarding polymer depolymerization.
[Edited on 27-6-2012 by 497]
|
|
|
SulfurApothecary
Harmless

Posts: 37
Registered: 26-6-2012
Location: Boise
Member Is Offline
Mood: For science!
|
|
It sounds very plausable condsidering that if it worked something like cracking petrol, it would seem viable, but I do not no, I can only speculate.
http://onlinelibrary.wiley.com/doi/10.1002/pol.1968.15006021... this is a link that would be vauluable if you had acess to it.
Polyethylene structure is like this, and it is formed by the polymerzation of ethylene.

Thermal decomposition/cracking would lead to this, which is ethylene:
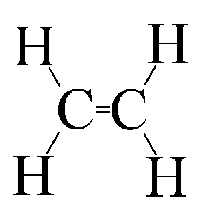
This would seem to work as the when the polymer was broken the carbon would be forced to make a double bond with the carbon next to it in order to
retain four bonds to each carbon.
[Edited on 28-6-2012 by SulfurApothecary]
[Edited on 28-6-2012 by SulfurApothecary]
You can't arrest me, it was for science!
|
|
|