franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Cyclohexitol, Cyclohexanehexol alias Inositol
A cyclohexane with six hydroxyls this sweet tasting isomer ( myo-inositol ) is sold as
a B vitamin in pharmacies and health food stores ( See sources listed at the bottom )
and as an antihypertensive pharmeceutical. It can also be a precursor for a variety
of possible explosive products that bear closer investigation. The only mention citing
a 1932 patent for the nitrate has been here _
http://www.sciencemadness.org/talk/viewthread.php?tid=890&am...
From the formula it is apparent that this has a positive oxygen balance. This means
that it can be blended into a plastic composition without diminishing the overall energy
product since the plasticizing substance will be combusted also rather than remaining
inert. A possible recipe for this could be a one to one molar ratio of the nitrate with
diethylene glycol or if more viscosity is desired , polyvinyl alcohol (-CH2-CH(OH)-)
would do as well , either one comprises only 12 percent the combined weight.
C6H6( NO3 )6 + C2H4( OH )2 => 6 CO2 + 2 CO + 6 H2O
But wait that's not all. There are in fact nine stereo-isomers of the molecule
scyllo-, cis-, chiro-, muco-, neo-,allo-, epi-, meso-,
and myo-inositol cis(equatorial)1,2,3,5-trans(axial)4-6 already discussed, shown here _
here is the phospate of this _
In PATR 2700 - The Encyclopedia of Explosives and Related Items by Federov
the description in Vol 7, I 109 , largely parrots the very dated patent already cited
and only speaks of a levelo and a dextral form , this is quite wrong. The sensitivity
assessment is not very reassuring , quote " [Inositol Hexanitrate] . . more impact
sensitive than Hexamethylenetriperoxydiamine , very sensitive to friction , and
explodes when ignited." The Chemistry and Technology of Explosives by Urbanski
Vol 2 page 200 , makes only a passing reference. Organic Chemistry of Explosives
by Agrawal & Hodgson on page 92 mentions briefly a related compound having
two nitro groups as well as four nitrates which is similarly sensitive.
http://www.chem.qmul.ac.uk/iupac/cyclitol/I6t10.html
http://www.iupac.org/publications/pac/1974/pdf/3701x0283.pdf
http://en.wikipedia.org/wiki/Inositol
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=892
http://www.ncbi.nlm.nih.gov/sites/entrez?term=892%5Bscid%5D%...
Funny this doesn't list tooth decay as a hazard 
http://physchem.ox.ac.uk/MSDS/IN/inositol.html
Scyllitol, 1,3,5/2,4,6-Hexahydroxycyclohexane
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=2489...
Has the structure pictured here , if you take the red atoms shown , to be the hydroxyls.

From this is derived an intriguing caged molecule , scyllo-inositol ortho-formate ester
http://en.wikipedia.org/wiki/Orthoformic_acid

Scyllo-inositol mono-orthoformate
Journal of Organic Chemistry , 1985 , vol 50 , page 4402
H. W. Lee, Y. Kishi,
Click Thumbnail for blowup
 
Download these ArgusLab files here _ http://www.badongo.com/file/4650709
The scyllo-inositol Di-orthoformate will form a box shape similar to cubane but
much more easily. This raises the question could this be peroxidated , forming
Scyllitol di-orthoformate hexaperoxide. C8H8(O2)6 => 8 CO + 4 H2O
The hexa-acetyl is a known compound and could just as easily be a peracetic ester
so the answer is not clear cut to me.
http://lb.chemie.uni-hamburg.de/static/CN/2_H_hexaa.php?cont...

The cis-inositol isomer has all it's hydroxyls in the equatorial plane and this makes it
useful for joining to acid nitroamines such as dinitrourea. The cis-inositol tri-dinitrourea
structurally resembles a boat propellor and would occur in two enantiomers.
C6H6(-NNO2.CO.NNO2-)3 => 3 CO2 + 6 CO + 3 H2O + 6 N2
- Click image for enlargement -

The Argus lab file is attached below
A prior related post is here _
http://www.sciencemadness.org/talk/viewthread.php?tid=6042&a...
U P D A T E
Tiraminoguanidine seen here can have its three amines ozonized forming nitroamine groups as I suggested in this other previous post _
http://www.sciencemadness.org/talk/viewthread.php?tid=6717&a...
Rosco Bodine posted on Triaminoguanidine here
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
http://www.sciencemadness.org/talk/viewthread.php?action=att...
The resulting di-acid compound can substitute for Dinitrourea or Methylene Dinitramine as above or alternatively to condense with
Tetrahydroxyquinone or Hexahydroxybenzene seen in the middle here below to give this other structure seen here _ - Click
image for enlargement -
[img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=19903[/img] 
The Argus lab files are here _
http://www.badongo.com/file/4664674
Preparation of Tetrahydroxyquinone
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p1011
Preparation of Hexahydroxybenzene
http://nvl.nist.gov/pub/nistpubs/jres/067/2/V67.N02.A06.pdf
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV5...
Applications in Supramolecular chemistry _ (scroll down to " esters and ethers "
http://www.rsc.org/delivery/_ArticleLinking/DisplayHTMLArtic...
PDF of the above ( see page 5 of PDF )
http://www.rsc.org/delivery/_ArticleLinking/DisplayArticleFo...
Related minor mention by ' garage chemist '
https://sciencemadness.org/talk/viewthread.php?tid=3751&...
CO reacts with potassium to form the potassium salt of hexahydroxybenzene
http://www.britannica.com/ebc/article-80880
Potassium reacts with carbon monoxide at temperatures as low as 60° C to form an explosive carbonyl (K6C6O6), a derivative of hexahydroxybenzene
A related post on this by ' JohnWW '
https://sciencemadness.org/talk/viewthread.php?tid=3751&...
http://www3.interscience.wiley.com/cgi-bin/abstract/10973052...
the same abstract _
http://dx.doi.org/10.1002/hlca.19640470604
Online sources of myo-inositol , available for just over 20 dollars a pound retail.
http://www.amazon.com/exec/obidos/search-handle-url/index=bl...
http://www.amazon.com/Jarrow-Formulas-Inositol-Powder-grams/...
http://www.amazon.com/Vitalabs-Inositol-Powder-8-oz/dp/B000R...
http://www.swansonvitamins.com/webapp/wcs/stores/servlet/Pro...
.
[Edited on 16-11-2007 by franklyn]
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have attempted a nitration of inositol once. The product was neutralized in solution, but still decomposed over the course of a few months.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Microtek, what nitration method did you use? I've tried the nitration of inositol but always recieved a gummy liquid product, though I've only used
70% HNO3 or a nitrate salt with H2SO4. The inositol in question is this overpriced product <a
href="http://www.vitaminking.com.au/shop_image/product/108d348d5a5190532f2d28bed664998a.jpg">here</a> and I am questioning its purity, the
picture shows a later version of the bottle, mine doesn't say "1g/g ORAL POWDER". I have found pure myo-inositol for a much cheaper price but I've put
off buying it due to the above reason.
I realise different optical isomers of inositol produce different products though I presume myo-inositol is the only inositol used as an food
ingredient.
[Edited on 27-9-2007 by Axt]
|
|
|
Mardec
Harmless

Posts: 33
Registered: 29-5-2007
Location: In a Lab
Member Is Offline
Mood: No Mood
|
|
What? inositol more sentitive then HMTD. I though in urbanski it was listed as similar to MHN..
I have 25 grams of inositol, not sure I want to nitrate it though..
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
From the little which I have read about inositiol hexanitrate , it is a fuse sensitive high explosive ,
a sort of material where the distinction is blurred
between primary explosive and secondary explosive ,
as this material DDT's very readily in small quantity .
My impression was it is very similar to DDNP but more powerful ....and a fair bit less storage and temperature stable ....
sort of in between HMTD and DDNP in that regard , but way more powerful on DDT than either of those comparisons .
Closest comparison material would
be MHN .
The use of dicyandiamide as a stabilizer would seem to apply here, as it applies to MHN ,
and though it hasn't been specifically described as useful IIRC , for stabilization of ETN as well .
[Edited on 27-9-2007 by Rosco Bodine]
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I don't remember the exact method I used, but I think I used Mega's method for MHN. Adapted to inositol obviously.
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I no longer have any idea what journal I got this from, nor do I know what its saying but heres an old French article on inositol hexanitrate.
Attachment: inositol hexanitrate.pdf (546kB)
This file has been downloaded 1215 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Plastic compositions made up of aliphatic nitrates has not been addressed much. I cited this classic 80 % PETN +
NG 20 % _
http://www.sciencemadness.org/talk/viewthread.php?tid=22911#... , Other similar compositions must also be practical.
Inositol Hexanitrate has been suggested as a material for boosters like PETN but having sensitivity more like ETN. Nitrated sugars , saccharides and
carbohydrates being solid characteristically exhibit sensitivity issues. Isopropyl Nitrate is less sensitive than other alkyl nitrates , alcohols ,
glycols and polyols. Blending 7 parts by weight ( 9.7 % ) of Isopropyl Nitrate which is oxygen deficient with 65 parts by weight of Inositol
Hexanitrate which has excess oxygen , comprises an oxygen balanced composition. This means that energy product should be the same as that of Glycol
Dinitrate ( 1500 Kcal/Kg ) but having relatively higher density and therefore a higher velocity of detonation. As a plastic material it should also
reduce sensitivity overall to ordinary handling , providing another alternative to those few already tried and documented.
6 C3H7NO3 + 13 C6H6(NO3)6 => 96 CO2 + 60 H2O + 42 N2
Another candidate is ethyl nitrate. 6 parts by weight ( 14.6 % ) of this with 35 parts by weight of Inositol Hexanitrate.
6 C2H5NO3 + 7C6H6(NO3)6 => 54 CO2 + 36 H2O + 24 N2
Myo-inositol 8 oz $ 13.49 + 4.99 shp
http://www.swansonvitamins.com/jarrow-formulas-inc-inositol-...
__________________________________________________
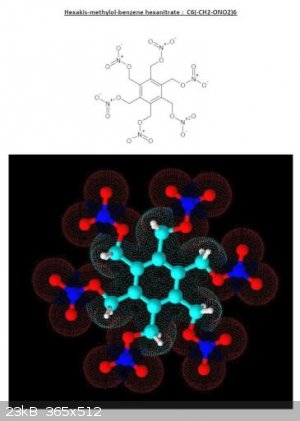
Hexamethylol Benzene Hexanitrate is a material closely related by structure. Addmixing 33 parts by weight of Hexamethylol Benzene Hexanitrate which is
oxygen deficient with 19 parts by weight ( 36.5 % ) of Methylene Dinitrate which has excess oxygen , comprises an oxygen balanced composition. This
concoction will likely be viscous syrup of middling density.
C12H12(NO3)6 + 4 CH2(NO3)2 => 16 CO2 + 10 H2O + 7 N2
http://www.sciencemadness.org/talk/viewthread.php?tid=1172&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=1172&a...
Both of the aforementioned cyclitols are highlighted here in a comprehensive overview. Pentaerythritol Tetranitrate is farther down on that same list.
http://books.google.com/books?id=WdmWkkKF1UoC&lpg=PA693&...
As one can see there is a large varied range of possible formulation compositions largely unexplored and untried.
P.S.
One last interesting point , the first formulation I proposed above with Isopropyl Nitrate , and the last one above here with Methylene Dinitrate ,
both have the same
molar distribution of detonation products. 49 % CO2 , 30 % H2O , 21 % N2.
Comparatively Gycol Dinitrate has the following distribution of detonation products : 40 % CO2 , 40 % H2O , 20 % N2
Given the greater amount of carbon burned by the composites , these will have a higher energy of explosion.
.
[Edited on 9-1-2015 by franklyn]
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Inositol Hexanitrate
I have wanted to do this nitration for a while now and have had this inositol for some time waiting to do and experiment with it so i attempted a
nitration of I-inositol. Using one of the hercules powder company patents as a guide which i found after a little searching. US 2340304.
I mixed up a nitrating bath in 50-50 each of distilled nitric and 98% sulfuric acids as the patent suggested.
So 30ml of each as to have a little more than required for 6g I-inositol i wanted to nitrate.
the mixed acids were put on an ice bath and bought down to 5C where i began the additions of the inositol in small portions -0.5g each addition over
the course of half hour wit the temp keeping between 7-11C as the patent also suggests and of coures with constant stirring. it was quite exothermic
and the powder really clumped together at first. After the 30 mins timeframe outlined in the patent the mixture was poured into 700ml iced water and
allowed to settle for 15 mins followed by filtration further washing with a mild Na carbonate solution, more washing and a quick recrystallization
from ethanol. i only poured the ethanol-IHN mix into water to precipitate for the moment since i ran out of time. again this was filtered with some
loss through the filter paper as extremely fine particles i just could not catch. dried and weighed the end result.
it is a very crisp white clumping solid of fairly low density so what i thought to be more was only A poor 4.4g! needless to say i wont be wasting
good nitric on further experiments with this compound unless it proves to be super special.. Although its high positive oxygen balance is usefull it
does seem to have similar sensitivity to MHN as a light tap of some IHN between steel surfaces did set it off with a loud report. it would be
interesting to see in an perfect oxygen balanced mixture with PETN or fueled up with some german dark Al powder.
I will be keeping an eye on it in regards to storage stability but reading a post by Microtek does not look too promising.
 
[Edited on 31-3-2015 by NeonPulse]
|
|
|