| Pages:
1
2 |
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Bromination of Propionic Acid in a Sealed Tube
What sort of pressures can be expected while heating propionic acid in a sealed tube to temperatures of 160C? This snippet seems to demonstrate that
alpha-bromopropionic acid can be prepared in two hours with strong heating.
I know there are other ways starting from alanine. I was interested in this route because I thought the acid halide had to be formed first in order to
break the resonance structure of the carboxylic acid.
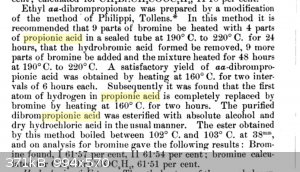
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Probably a couple of atmospheres or so... my concern would be the vapor pressure of the bromine, which would be at something like 30-35 atmospheres.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
One can apparently avoid heating by using UV light (reference: see, for example, https://pubs.acs.org/doi/abs/10.1021/ja01604a013?journalCode... ):
Br2 + hv --> .Br + .Br
RH + .Br --> .R + HBr
.R + .Br --> RBr
.R + RH --> R-R + .H
.H + RH -->.R + H2
.H + .H --> H2
.Br + Br- = .Br2- (assumes water vapor creating bromide)
R-R --> Products
....
You will, however, I suspect get a larger mix of products and the HBr as gas could increase pressure in the tube. Note, in the historical procedure
cited, HBr gas is removed so the vessel is NOT sealed.
Also, heating Br2 to high temperature can also induce radical formation, but I would not like to be holding that tube if it explodes from the pressure
buildup!
Do test the safety of the photolysis approach using a small amount as, for example, H2 + Cl2 (unclear with respect to Br2, a source claims the need of
a flame, not photo-sensitive, see http://uki.vdu.lt/wp-content/uploads/sinergija/EN/chemija/ch...) can be made to explode with sunlight or red light. Also, in the presence of H2,
O2 and Cl2 a light activated explosion (see https://www.nature.com/articles/127853a0 ) and perhaps also with Br2, so no oxygen presence. Also, heating H2 and Br2 to a high temperature will
cause an explosion (see https://pubs.acs.org/doi/abs/10.1021/ja01604a013?journalCode... ) so removing gases, not just HBr but some created H2, from the mix is likely
required to avoid an explosion at elevated temperatures.
[Edited on 14-9-2018 by AJKOER]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
How come most preparations of alpha-halocarboxylic acids use phosphorus? I know that mechanism includes the acid halide, followed by halogen addition
at the alpha-carbon.
Are there issues with this approach? Less extreme conditions I guess....
Thank you, AJKOER!
EDIT: The chance of explosion is sort of off-putting.
[Edited on 14-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Does anyone know if lactic acid responds like a secondary alcohol to Lucas' reagent?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Maybe, why not? Perhaps it is easier than alternate routes.
There are a number of examples of @Chloro-propionic esters, being synthesized from lactic acid esters.
Diazotization/substitution of alanine, can produce a pure enantiomer. But, if your final product doesn't have a center of asymmetry; the
cheapest, easiest route to synthesis, should prevail.
https://chemistry.mdma.ch/hiveboard/chemistrydiscourse/00046...
https://the-hive.archive.erowid.org/forum/showflat.pl?static...
Lactic acid is cheap, cheap, cheap. Err... I mean, inexpensive.
https://ingredi.com/l-lactic-acid-88-55-lbs-pail/?gclid=EAIa...
[Edited on 14-9-2018 by zed]
[Edited on 14-9-2018 by zed]
[Edited on 14-9-2018 by zed]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JJay  | | Probably a couple of atmospheres or so... my concern would be the vapor pressure of the bromine, which would be at something like 30-35 atmospheres.
|
My concern would be the pressure from the hydrogen bromide produced.
How would you safely open the tube?
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by JJay  | | Probably a couple of atmospheres or so... my concern would be the vapor pressure of the bromine, which would be at something like 30-35 atmospheres.
|
My concern would be the pressure from the hydrogen bromide produced.
How would you safely open the tube? |
I am not sure yet at this point. I think it might be better to use some sort of steel pipe with caps that can be screwed on and off. Or better yet,
valves with hosing that can be opened to relieve pressure several feet away.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by Loptr  | | What sort of pressures can be expected while heating propionic acid in a sealed tube to temperatures of 160C? |
Wouldn't that depend on the size of the tube? So...the vapor pressure of propionic acid is the only consideration here...
So which steel is it at the hardware store that doesn't react with the acids at 160C?
If only there were bromopropionic syntheses already posted. Funny how no one ever thought of that before the forum turned to nearly total garbage.
[Edited on 15-9-2018 by S.C. Wack]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by S.C. Wack  | Quote: Originally posted by Loptr  | | What sort of pressures can be expected while heating propionic acid in a sealed tube to temperatures of 160C? |
Wouldn't that depend on the size of the tube? So...the vapor pressure of propionic acid is the only consideration here...
So which steel is it at the hardware store that doesn't react with the acids at 160C?
If only there were bromopropionic syntheses already posted. Funny how no one ever thought of that before the forum turned to nearly total garbage.
[Edited on 15-9-2018 by S.C. Wack] |
There are several synthesis of alpha-bromopropionic acid posted to this forum. Very presumptuous of you to assume that I was only interested in the
production of alpha-bromopropionic acid. I believe that I stated the purpose for my question in my initial post.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
How exactly is it presumptuous when that is the first time you mention anything other than the a-bromo acid, and in your first post it's clear that
you are asking about a single sentence in your google snippet, the one dealing with alpha-haloacid, ignoring the main dibromo thrust...ha...I don't
feel presumptuous...
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by S.C. Wack  | | How exactly is it presumptuous when that is the first time you mention anything other than the a-bromo acid, and in your first post it's clear that
you are asking about a single sentence in your google snippet, the one dealing with alpha-haloacid, ignoring the main dibromo thrust...ha...I don't
feel presumptuous... |
| Quote: | | I know there are other ways starting from alanine. I was interested in this route because I thought the acid halide had to be formed first in order to
break the resonance structure of the carboxylic acid. |
I stated the fact that I was aware of other routes in the first sentence. The second sentence adds that I am interested in the route because of the
odd addition which usually requires the acid halide being formed first. I was hoping this could extend to other carboxylic acids than just propionic
acid. The interest was in the bromination reaction itself.
[Edited on 15-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by JJay  | | Probably a couple of atmospheres or so... my concern would be the vapor pressure of the bromine, which would be at something like 30-35 atmospheres.
|
My concern would be the pressure from the hydrogen bromide produced.
How would you safely open the tube? |
I haven't tried this, and the vapor pressure of the hydrogen bromide is certainly a hazard, even after the tube has cooled, but I think the vapor
pressure of hydrogen bromide is considerably reduced when it is dissolved in carboxylic acids.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by zed  | Maybe, why not? Perhaps it is easier than alternate routes.
There are a number of examples of @Chloro-propionic esters, being synthesized from lactic acid esters.
|
It looks like 2-chloropropionic acid is miscible with water... I wonder if lactic reacts with Lucas reagent in the manner typical of a secondary
alcohol. If it does... is the product hard to salt out....
Oh and definitely take a look at the toxicity of 2-chloropropionic acid if you're thinking about making some. I don't suggest producing gallons of it
in your garage.
[Edited on 15-9-2018 by JJay]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by JJay  | Quote: Originally posted by zed  | Maybe, why not? Perhaps it is easier than alternate routes.
There are a number of examples of @Chloro-propionic esters, being synthesized from lactic acid esters.
|
It looks like 2-chloropropionic acid is miscible with water... I wonder if it reacts with Lucas reagent in the manner typical of a secondary alcohol.
If it does... is it hard to salt out....
Oh and definitely take a look at the toxicity of 2-chloropropionic acid if you're thinking about making some. I don't suggest producing gallons of it
in your garage. |
Yeah, a while back I read some where about it being a neurotoxin. Thanks for the heads up, though.
I have some DL-alanine, and plan to buy some lactic acid. I think it does behave as a secondary alcohol because it does participate in substitution
reactions with halo acids to some extent, or at least that is what I recall from past google searches.
[Edited on 15-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
If you acquaint yourself with the mechanism of the Lucas reaction, you will see why it will not work with lactic acid or esters.
I find myself in partial agreement with SC Wack regarding some of the stuff posted here. Loptr posted a reasonable question. However, some of the
follow-on comment is just speculative based on a shallow knowledge of some aspects of chemistry. This sort of thing happens all across this forum. A
bit of searching and deeper "book learning" would go a long way to eliminating some of the nonsensical posts that appear all too frequently. Perhaps
this is not the best place to post my rant, but is something that has concerned me for a long time and SC Wack lit the fuse.
AvB
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by AvBaeyer  | If you acquaint yourself with the mechanism of the Lucas reaction, you will see why it will not work with lactic acid or esters.
I find myself in partial agreement with SC Wack regarding some of the stuff posted here. Loptr posted a reasonable question. However, some of the
follow-on comment is just speculative based on a shallow knowledge of some aspects of chemistry. This sort of thing happens all across this forum. A
bit of searching and deeper "book learning" would go a long way to eliminating some of the nonsensical posts that appear all too frequently. Perhaps
this is not the best place to post my rant, but is something that has concerned me for a long time and SC Wack lit the fuse.
AvB |
Ok, this is what confuses me. Is lactic acid a secondary alcohol? If so, then an SN1 substitution could be take place in a Lucas type reaction.
Secondary alcohols prefer SN1 reactions, so where is the issue?
EDIT: I will read up on the mechanism some more and see if I can find the issue...
[Edited on 15-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
The issue is the Sn1 mechanism with a carbonyl group attached to the carbon bearing the incipient positive charge. Consult a good organic chem text
which discusses mechanisms.
AvB
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by AvBaeyer  | If you acquaint yourself with the mechanism of the Lucas reaction, you will see why it will not work with lactic acid or esters.
|
I just spent some time looking at the mechanism, and now I am completely baffled... the Lucas reaction doesn't work with lactic acid??
I really must be missing something important here... I'm going to have to study the mechanism further to make sense of this curious non-reactivity.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Ahh, carboxyl is electron withdrawing. The carbocation is stabilized by donors. The carbocation intermediate isn't very stable.
So in this case we see elimination rather than substitution? If so, you should get acrylic acid. Halo acid addition leads to 3-halopropionic acid
through anti-Markovnikov addition being an a,b-unsaturated acid. Right?
[Edited on 16-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Loptr  | Ahh, carboxyl is electron withdrawing. The carbocation is stabilized by donors. The carbocation intermediate isn't very stable.
|
Oh, of course! I was looking at how easy it is for lactic acid to form a carbocation, not the stability of the carbocation. It can form a carbocation
easily, but the carbocation is extremely reactive, so I guess it doesn't exist long enough to react with a halogen anion.
Markovnikov addition places the halogen on the most heavily substituted carbon, so if you added anhydrous hydrogen bromide to acrylic acid (in the
absence of UV, peroxides, etc. that could form radicals), you would get your desired product.
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Actually, there seems to be an exception with a,b-unsaturated carboxylic acids. The addition is opposite of that seen with alkenes due to the electron
withdrawing group.
https://www.quora.com/What-product-is-formed-on-reaction-of-...
I am trying to find the relevant chapter in my text book.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In the high-pressure method, I think that bromination of the acyl hypobromite might occur. This intermediate is enolizable.
Consider instead using a sulfonyl halide with lactic acid or its esters. In some jurisdictions, tosyl chloride may be available. If not,
methanesulfonyl halides can be made by halogenation of methanesulfinate salts, which are available from the Birch reduction of methylsulfonylmethane
as described here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=91...
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by clearly_not_atara  | In the high-pressure method, I think that bromination of the acyl hypobromite might occur. This intermediate is enolizable.
Consider instead using a sulfonyl halide with lactic acid or its esters. In some jurisdictions, tosyl chloride may be available. If not,
methanesulfonyl halides can be made by halogenation of methanesulfinate salts, which are available from the Birch reduction of methylsulfonylmethane
as described here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=91... |
Yeah, that is a good idea. I had thought about the sulfur halide approach since it can be used to prepare chloroacetic acid. There is also the route
that uses PPA and Br2.
EDIT: Hypobromite. That's what I was looking for!
[Edited on 16-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You're right... it's a stability issue. If I'm understanding this correctly, other parts of an intermediate molecule pull away the halogen cation.
The Lucas reagent won't react with lactic acid in the typical manner for a similar reason. Right?
|
|
|
| Pages:
1
2 |