nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Preparing sodium bisulfite solution
I have several sodium salts, such as sulfate (Na2SO4), bisulfate (NaHSO4), metabisulfate (Na2S2O6) and thiosulfate (Na2S2O3.5H2O).
Unfortunately, this time I need sodium bisulfite ideally as 10% aq. solution.
I found one possible preparation by carefully dissolving sodium metabisulfate in water:
Na2S2O5 + H2O <-> 2NaHSO3
This reaction is two-way and I am not sure how shift balance to the right side. Adding a base? Increasing concentration?
Here is one recipe for making sat. sodium bisulfite:
| Quote: |
1. Add 4.75 g of sodium metabisulfite (di-sodium disulfite, Na2S2O5) to 6.25 mL of sterile, degassed ddH2O.
2. Add 1.75 mL of 2 m NaOH.
3. Add 1.25 mL of 1 m hydroquinone (0.11 g in 1 mL of H2O).
4. Heat to 50°C in the dark, inverting the tube frequently.
5. Adjust the pH to 5.0.
|
This seems overcomplicated for my purposes (I need just a dilute solution). It seems the addition of base favours sodium bisulfite to be generated,
then neutralized with hydroquinone (which will form precipitate and can be filtered out). I don't understand the last step of adjusting pH. Why?
Of course I could make the solution the traditional way, by bubbling SO2 through a solution of sodium carbonate, which is the standard method, but it seems too laborious as:
- the SO2 gas generator and bubbler need to be constructed
- the resulting bisulfite aqueous solution has to be fully evaporated in vacuo, washed and dried
Maybe the easier way (not involving toxic gases) would be to prepare the saturated solution first, using sodium metabisulfate, NaOH, hydroquinone and
perhaps little HCl and then remove water from this under vacuum. However I am not sure about metabisulfate contamination. This probably has have
precise stoichiometry. Or doesn't?
Anyway, having the bisulfite crystals, I can then easily prepare solution at whatever concentration needed.
The compound seems to be unstable in both aqueous environment and dry so I have to find a way to prepare it on demand anyway, rather then just buying
it. I plan to store the crystals in sealed contained with a desiccant.
[Edited on 22-9-2018 by nimgoldman]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I am extremely reluctant to answer this question because a) this subject would be taught in the first week of general chem and b) you used the word
"recipe." So I'm just going to tell you how to figure out the answer:
1. Figure out how much sodium bisulfite you need for an 10% aqueous solution. Call that quantity A.
2. Look up the molecular weight of water (call it b) and the molar mass of sodium metabisulfite (c).
3. Figure out how much water you would need (D) if you were making an 10% aqueous solution of sodium bisulfite, starting with sodium bisulfite rather
than sodium metabisulfite.
4. Now measure out D units of water. Add A * b / (b / 2 + c) / 2 units of water. Add A * c / (b / 2 + c) units of sodium metabisulfite. Stir to
dissolve.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Quote: Originally posted by JJay  | I am extremely reluctant to answer this question because a) this subject would be taught in the first week of general chem and b) you used the word
"recipe." So I'm just going to tell you how to figure out the answer:
1. Figure out how much sodium bisulfite you need for an 10% aqueous solution. Call that quantity A.
2. Look up the molecular weight of water (call it b) and the molar mass of sodium metabisulfite (c).
3. Figure out how much water you would need (D) if you were making an 10% aqueous solution of sodium bisulfite, starting with sodium bisulfite rather
than sodium metabisulfite.
4. Now measure out D units of water. Add A * b / (b / 2 + c) / 2 units of water. Add A * c / (b / 2 + c) units of sodium metabisulfite. Stir to
dissolve.
|
I of course know how to make solutions and convert moles to grams but that's not the question...
My problem is the chemistry of it: even if I dissolve the metabisulfite in water, how do I ensure it will become a solution of bisulfite and
not (partly) a solution metabisulfite. The equation is two-way so I am trying to realize what shifts the balance towards bisulfite. Changing
pH? Temperature? Light? Concentration?
It is known that sodium bisulfite spontaneously decomposes to sodium sufite, sulfur dioxide and water. This happens faster in aqueous environment so
this is the reason why I am concerned about the simple preparation by just dissolving the metabisulfite.
The reason I added the word "recipe" is because the linked website uses that exact wording. I ask precisely because I want to know how the
principles in the recipe works, not being handed one (as obviously I already have three).
Sorry for all that bolded text, but I want to make myself clear. I understand you might have skimmed the post and I might have just post the core
question.
The recipe (see link) uses strong base (NaOH), organic acid (hydroquinone) and calls for possibly another acid to adjust pH. I would like to
understand why all these chemicals are needed if all that is required is just dissolving metabisulfite in water...
Maybe this is covered in basic chemistry, I am learning on the go but the acid-base chemistry somewhere in the middle of my Zumdahl and I am slowly
getting there to get my theoretical background...
[Edited on 22-9-2018 by nimgoldman]
[Edited on 22-9-2018 by nimgoldman]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The equilibrium usually substantially favors the bisulfite ion... according to this article, the equilibrium constant varies by the ionic strength of
the solution: https://www.sciencedirect.com/science/article/pii/S002235491...
It sounds like you know more about this subject than I do.
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
found here https://www.finishing.com/596/91.shtml I found this
|
|
|
JScott
Hazard to Self
 
Posts: 51
Registered: 23-8-2018
Member Is Offline
|
|
"I am extremely reluctant to answer this question because a) this subject would be taught in the first week of general chem and b) you used the word
"recipe." So I'm just going to tell you how to figure out the answer:"
This is why I don't ask more questions than I do. For me, not so big a deal. I have a lot time to read anyhow, and my expectations are realistic. But
I wonder how many 'new kids' are put off by this type of elitism?
Oh, and I am currently auditing four first year chem classes (online) and this is far from first week material (but you knew that, you were just being
mean). At least at Purdue, UCI, MIT, and Nottigham (sp). In fact I can't believe they are going over stuff I did eighth grade. How frustrating! Pay
all of that money to be lectured about the states of mater and the importance of scientific notation. I can see why kids are bored!
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Sodium metabisulfite is an anhydrous salt and will react fully with a molecule of water to form two equivalents of sodium bisulfite. You can't have a
solution of metabisulfite; it will only exist as bisulfite.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Quote: Originally posted by Amos  | | Sodium metabisulfite is an anhydrous salt and will react fully with a molecule of water to form two equivalents of sodium bisulfite. You can't have a
solution of metabisulfite; it will only exist as bisulfite. |
That's not exactly true.
When dissolving sodium metabisulfite in water, certain amount of SO2 gas is released. It is because following reaction happens:
2NaHSO3 -> Na2SO3 + SO2 + H2O
hence certain amount of bisulfite is lost and the solution is contaminated with sodium sulfite and SO2.
So I am trying to find out what factors affect the decomposition (probably temperature, pH and concentration).
Note that SO2 production is the exact reason why winemakers use metabisulfite for sterilization! But of course I don't want that in solution, just the
bisulfite.
To make things more perplexing, some chemical suppliers provide "sodium metabisulfite solution" even though metabisulfite cannot exist in a solution.
However, the vendor states that a synonym for sodium metabisulfite is simply "sodium bisulfite anhydrous". This makes sense as drying bisulfite yields
metabisulfite and vice versa.
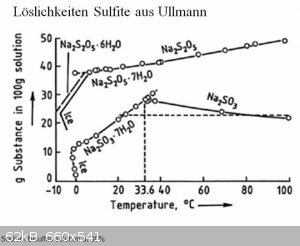
These are solubility curves for sulfites. It seems 10-30% bisulfite solution can be easily made even with ice cold water.
I guess there is only very small amount of side products created if the dissolution of metabisulfite in water is done slowly especially if the water
is cold.
[Edited on 23-9-2018 by nimgoldman]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by nimgoldman  | When dissolving sodium metabisulfite in water, certain amount of SO2 gas is released. It is because following reaction happens:
2NaHSO3 -> Na2SO3 + SO2 + H2O
hence certain amount of bisulfite is lost and the solution is contaminated with sodium sulfite and SO2.] |
That sounds like MSDS chemistry. Plus, bisulfite is no different.
I would think that metabisulfite solution is assayed in either %SO2 or MHSO3, and that the residue of vacuum evaporation of a solution of
metabisulfite is metabisulfite.
AFAIK metabisulfite's reactions are those of the solid, and bisulfite is the solution. I never hear anyone say metabisulfite anion exists in solution.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
So far it seems that simply dissolving metabisulfite in cold water, while stirring, produced pure enough solution of bisulfite.
However, I don't know whether this can be used in organic synthesis (e.g. for aldehyde purification) since the SO2 and Na2SO3 contaminations might
cripple the reaction. But maybe I am worried unnecessarily.
[Edited on 24-9-2018 by nimgoldman]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Your worries indeed seem unnecessary. The equilibrium you speak of is there but it doesn't seem to interfere with bisulfite adduct formation; many
amateurs just use a stoichiometric excess of metabisulfite or bisulfite to prepare one. A cold solution of bisulfite won't give off appreciable sulfur
dioxide, either. Start boiling one though and everyone in the room will notice the smell very quickly.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Anything calling for bisulfite is calling for acidic SO2. The only difference between sulfite and bisulfite is pH, and all that is is how much SO2
you're putting in.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
I see, thanks! This clears things up a lot.
|
|
|
jus_thom
Harmless

Posts: 7
Registered: 22-2-2019
Member Is Offline
|
|
Amount of SO2 released from Sodium Bisulfite solution
Hi everyone,
I ran into this post while researching on Sodium Bisulfite and I think this post might be able to help me. Apologies in advance if this is not the
right way to post a query.
I am currently working on determining the amount of SO2 that would be released in a Sodium Bisulfite chemical storage room. The tanks usually vent
outside the building, so the only credible scenario (in my opinion) is if you ever had a spillage from the tank (via overflow pipework).
So, my question is how do i determine the amount of SO2 that would be released from a known spillage volume (lets say 1m3) of 40% w/w Sodium Bisulfite
solution at room temperature and atmospheric pressure?
|
|
|