RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Recrystalizations - are some fractions more pure?
I am wondering if when doing recrystalizations if whether some parts of the crystals would be more pure. For example if dissolving a compound that
increases solubility with temp linearly by say 200g / 10 deg C, so at BP 1L of water would hold 2KG of compound, 90C would hold 1.8kg, 80C 1.6kg, etc
down to say 20C - 0C where it still holds much of the salt.
I'm wondering if the crystals were filtered out at each 10 degree interval, if the crystals from the highest temp that are the first to crash out,
would be the purest, or if crystals towards the bottom of the solubility would be purer. Or if that makes no difference at all.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Depends on what you are trying to get away from (more soluble or less soluble impurity). I've seen plenty of processes which cool to say room
temperature and filter and advise against going lower to prevent collecting garbage. I've seen others take something close to the freezing point of
methanol and eek out every bit. Some processes boil down the solvent and go after multiple crops. I know you've heard of each of these situations
but they're similar to what you're describing already. I have seen only a fickle few processes actually use a fractional crystallization in
production. This is due to how infrequently it comes up coupled with the fact that as you're doing the filtration more solids are likely to crash out
so you end up heat tracing all your lines which is a pain in the butt. Usually though they avoid this by just adding more solvent on the front end so
they can take a crop at room temperature and then cool to negative whatever to get a second crop.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Too many factors to make generalizations.
Slower crystallization tend to be more pure in my experience.
Hotter is better from some crystallizations, others colder is better.
And still others best occur with a temperature change, generally cooling the mix.
The solubilities of impurities at different temperatures make a big difference.
If your impurity is much more soluble at high temperatures then doing it hot is preferable.
If your product is much more soluble at high temperatures then doing it cold or with a temperature change is going to be preferable.
In your case with a temperature change crystallization, it depends on the impurity.
If the impurity has a similar solubility slope but is more soluble then first crystals will be more pure.
If it is less soluble then later crystals will be more pure as the impurity will crystalize first.
Again, it depends on your impurities.
For example I have a salt with an impurity.
The impurity is much more soluble at high temperature (ie. boiling) but the product and impurity are similar at room temperature with the impurity
being more soluble. So I am going to have to do the crystallization just below boiling and slowly evaporate then filter hot.
I know I am not going to get total separation and have an additional reaction planned where the new product will be much less soluble. So I can
crystallize that product and get a much cleaner end result.
[Edited on 23-9-2018 by macckone]
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=20352
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Thanks for the replies. It makes a lot of sense that it is all dependent upon the solubility curves of the primary salt and the contaminates.
I was trying to purify some CuSO4. It was made from copper wire, H2SO4 and H2O2 so I'm guessing it is pretty pure as is, but I thought I'd do a few
recrystalizations and see if there was any difference, though I'm not sure how I'll test it, but I'm doing it to practice the process. I'm also
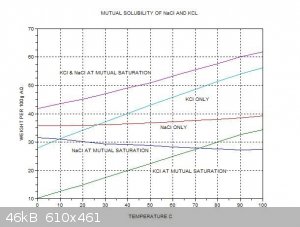
trying to purify some 95/5 KCl/NaCl which the above thread has a lot of good information on. I found the one chart really helpful.
I just had a really odd reaction while pouring some boiling CuSO4 into the filter. It started boiling furiously, almost like a run-away reaction upon
hitting the funnel which had a cotton filter. I'm not sure why this happened, I didn't think the solution was even saturated but the way it started
to form very fine crystals within the funnel, it seems to have been super-saturated.
Out of curiosity, how much extra salts can a super saturated solution hold? I've found a wide range of numbers for the amount of CuSO4x5H2O that can
be dissolved a solution at 100C, from ~130g/100ml, 215g/ml and numbers in and around those. I can verify that I was able to dissolve ~215g/100ml,
possibly more.
[Edited on 9-24-2018 by RogueRose]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yes! We have no bananas!
A lot of factors come into play during crystallization, and there is no one answer.
The general rule is: The faster and smaller, Crystals are formed, the purer they are.
Sadly, this results in a huge total surface area on these small crystals, and crud clings to this huge surface area. Further, good luck rinsing 'em
off. Those tiny crystals tend to re-dissolve. Moreover, they may be infinitely pure, but very very tiny. Good luck filtering something like that.
Colloidal.
So, even though larger crystals may contain inclusions, they may be easier rinse off, and otherwise deal with.
[Edited on 25-9-2018 by zed]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by RogueRose  | | Out of curiosity, how much extra salts can a super saturated solution hold? I've found a wide range of numbers for the amount of CuSO4x5H2O that can
be dissolved a solution at 100C, from ~130g/100ml, 215g/ml and numbers in and around those. I can verify that I was able to dissolve ~215g/100ml,
possibly more. |
I made up copper sulphate solutions many times when I was experimenting with electro-chemical cells,
when I tried to make saturated solutions
they always looked less intense colour than many that I've seen on the 'net
but solubility was about that shown in Wikipedia.
So;
how did you manage to dissolve c215g/100ml ?
Was the solution transparent, or was there cloudiness, due to suspended/colloidal CuSO4 ?
......................................................................
My re-formatted Wikipedia solubility spreadsheet
Attachment: AlphabeticalSolubilityOfSalts.ods (55kB)
This file has been downloaded 274 times
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
I started by weighing out about 1KG of CuSO4 then I added about 100ml per 300g CuSO4 and after boiling for about 2 hours on low, there was still some
salt undissolved, do I added about 33-50ml water and the rest of the salts dissolved. No more salts on the bottom or undissolved in solution. I
allowed a strong boil for about 30-40 mins until salts started to drop out again and then poured the solution into a funnel w/ filter then placed the
funnel and beaker into the microwave to heat while it filtered.
The solution is an extremely dark solution and I think it's bp is well above 100C but I don't have a reliable thermometer ATM. An IR thermometer said
151F but I didn't believe that. When saturated the solution bumps and burps and sounds like it is an angry volcano and I didn't want to be anywhere
near it it was shaking and almost jumping off the hot plate. I'm glad I was using extra heavy walled filtering flask w/ wide mouth or I wouldn't have
touched it.
I really have no idea what the final concentration was but I would say it was well above 215g/ml and 250 to almost 300. I was very surprised at the
end. I would fill an 8oz beaker after filtering and after the liquid cooled there may have been 1 - 2 oz of liquid that was poured off the room temp
crystals (would have been less liquid if frozen).
I will try to run a process to complete evaporation of water and see what numbers I get but that still is a different result than dissolving a salt
into a solvent, than how much salt a solvent will hold.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
This is the exact opposite of my (limited) understanding.
I thought that the purest crystals are obtained by slow growth of well shaped large crystals.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I agree with Sulaiman. My understanding is that fast crystal formation can trap impurities in the crystal, while very slow growth much more
effectively excludes them. Very slow cooling (i.e. insulated flask) or slow evaporation is the way to go.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Once upon a time, I studied this matter fairly extensively. I am transmitting the zeitgeist of 50 years ago. There were contrary opinions even
then, and all factors aren't equal.
Bigger, smaller, purer, slower, faster.... These are theoretical considerations. What works, works. To a certain extent, growing crystals is
voodoo-ish.
It is most important, to find a practical procedure that works for what you wish to achieve.
What do you need?
https://www.youtube.com/watch?v=k3nClG-DT7w
[Edited on 25-9-2018 by zed]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
faster smaller vs slower larger depends as much as anything else on the actual substances involved. Some impurities will crystallize with the crystal
forming compound creating inclusions, others won't. You really need to know what the impurities are. If your impurity will form inclusions then you
want smaller crystals. If your impurity does not crystallize with the compound then you want large crystals. For example sodium and potassium chloride
will form co-crystals. Other combinations are going to be different and I can't even provide a set of rules as crystallization is very complex.
copper sulfate will grow nice large crystals with minimal impurities using a slow evaporation provided it is reasonably pure to begin with. With
copper sulfate you can get visible disruption of the large crystals if impurities get included resulting in smaller crystals with side growths.
|
|
|
SHlTnipplesmadeofeggs
Harmless

Posts: 8
Registered: 26-9-2018
Member Is Offline
|
|
Quote: Originally posted by RogueRose  |
I just had a really odd reaction while pouring some boiling CuSO4 into the filter. It started boiling furiously, almost like a run-away reaction upon
hitting the funnel which had a cotton filter. I'm not sure why this happened, I didn't think the solution was even saturated but the way it started
to form very fine crystals within the funnel, it seems to have been super-saturated.
[Edited on 9-24-2018 by RogueRose] |
I'm not going to attempt to answer your other questions as they seem to have mostly been answered, but I believe with this (there is a term for this
but I can't remember) its where when a liquid is heated and perhaps to its boiling point but isn't vaporizing much, shock can make it "explosively
boil" (maybe that's the term)
I witness this often when making tea concentrate, I boil 2 cups of water in microwave for like 4 or 5 minutes. When I pull it out there are only a few
bubbles, but when I put in the tea bags I have it actually boil so hard it shot out of the measuring cup (I heat for less time now).
This is a rather shit explanation and I'm sure someone who knows more will explain it better, but there ya go.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Superheating?
https://www.snopes.com/fact-check/boil-on-troubled-waters/
Which is why we use boiling chips on occasion. To provide nucleation sites for the liquid/gas transition.
Perhaps when crystals started to form, the crystals provided those sites.
Though I'm curious, is it possible that the crystal formation itself, could be exothermic enough to cause the water to boil?
And, a cotton filter ..... So many factors.
[Edited on 4-10-2018 by zed]
[Edited on 4-10-2018 by zed]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
bumping is a special case of superheating.
superheating will suddenly boil if the liquid comes in contact with pretty much anything.
In bumping the situation is more complex because the heat is generally introduced at the bottom of the liquid which is at a slightly higher pressure.
When the substance starts boiling a small amount of liquid becomes a huge amount of vapor. Higher viscosity and density means there is less
convection increasing the tendency to bump.
Example, in water 18ml of water becomes 22.4L of gas which has an expansion factor of over 1200
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | | Though I'm curious, is it possible that the crystal formation itself, could be exothermic enough to cause the water to boil? |
I was thinking the exact same thing. All it would really need is to be a little exothermic to at least magnify the bumpfroth effect that you get when
you dump boiling liquid into a filter.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
In order for the crystallization to be that exothermic you would need a lot of energy to get a substance to go into solution. That means dissolving
in the first place would be difficult and it would be unstable. Generally crystallization from solution absorbs energy or releases very little.
All that said, it is hypothetically possible if you have a solvent with a low enough heat of vaporization and the solution would have to be
supersaturated.
[Edited on 5-10-2018 by macckone]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Exothermic crystallization is well documented
https://en.wikipedia.org/wiki/Sodium_acetate#Heating_pad
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
A lot of times when you hot-filter a boiling solution (saturated or otherwise) just pouring it right into a filter (pre-heated or otherwise) is enough
to cause the phenomenon that has been described: a sort of vigorous boil, clearly more energetic than what was seen before the liquid hits the filter.
It would only need to be a slightly exothermic crystallization to to enhance this effect significantly. Moreover, there are quite a few substances
that crystallize exothermically. I would hardly call it uncommon, I think it probably isn't noticed as much because crystallization often happens
slowly and when we aren't monitoring internal temperature. The few times I have witnessed it while stirring with a thermometer, I have been quite
amazed at how much the temperature can rise and how quickly.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Unionized: that is supersaturated, nor does it release a lot of heat. In fact releasing a lot of heat would be a problem since people are putting it
on their skin. The question I was responding to was related to crystallization boiling the solvent and that is only likely in a supersaturated
solution with a low heat of vaporization solvent.
Happyfooddance: see my comment about supersaturated. if the substance is already at boiling, it isn't providing much heat. the filter is going to
provide a lot of new nucleation sites as well. You can also get this effect pouring pure water through a filter or adding a tea bag to a cup of
boiling water. And putting a tea bag in water is not likely to release any significant amount of energy.
[Edited on 6-10-2018 by macckone]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I do not know, and have not tried to calculate, the heat released
when a teabag is put into microwave super-heated water,
but it has scared me more than once 
Even a thermometer may initiate violent boiling so experimenters take care.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
"Suppose that we heat one kilogram of water from 100 °C (its normal boiling temperature) to 101 °C, i.e. it is now superheated by 1 °C. When it
begins to boil, it will very quickly cool to 100 °C, and the heat liberated turns water into steam. Cooling this kg of water by 1 °C gives 4.2 kJ,
which is enough to evaporate c/L = 4200/2230000 kg of water. This is only 1.9 millilitres of water, which does not sound very much, but it turns into
3 litres of steam. Those three litres of steam are created inside the hot water, quite suddenly, so the water is ejected violently from the
container."
http://www.animations.physics.unsw.edu.au/jw/superheating.ht...
|
|
|